Determination of 2,4-dinitrophenylhydrazine using carbon paste modified with nanoparticles by cyclic voltammetry, high-performance liquid chromatography and spectrophotometry methods
Volume 6, Issue 03, Pages 19-35, Sep 2023 *** Field: Analytical Method in Chemistry
Abstract
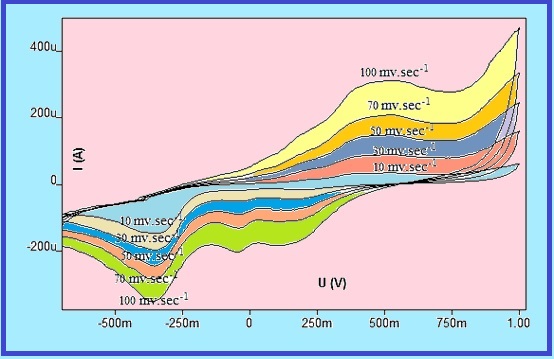
The research deals with the manufacture of an electrode using modified carbon paste to determine 2,4-dinitrophenylhydrazine (2,4-DNPHZ), The modified carbon paste electrode (NiO-NCQD/g-C3N4/MCPE). The results show the presence of oxidation and reduction peaks, and it is subject to a quasi-reversible system; the best value of pH is (1) using sulfuric acid with a concentration of (0.1M), and scan rate is 100 mv sec-1, it was linearity range of (1-1000) µM for oxidation, and (100-1000) µM for reduction, with correlation coefficient (R2=0.9717) and (R2=0.9914) for each of them, respectively. The proposed electrochemical method was compared with two methods they are spectrophotometry at a wavelength 360) nm( and high-performance liquid chromatography (HPLC) at wavelengths (340 and 250) nm. It turned out that the electrochemical method (NiO-NCQD/g-C3N4/MCPE) was superior to the spectrophotometry method in terms of the detection limit. It turns out that there is no significant difference between (HPLC) and (NiO-NCQD/g-C3N4/MCPE) in terms of accuracy. The proposed electrochemical method is a new analytical method characterized by accuracy, repeatability, and reliability.
References
W. A. Adeosun, A. M. Asiri, H. M. Marwani, Real time detection and monitoring of 2, 4-dinitrophenylhydrazine in industrial effluents and water bodies by electrochemical approach based on novel conductive polymeric composite, Ecotoxicol. Environ. Safe., 206 (2020) 111171. https://doi.org/10.1016/j.ecoenv.2020.111171
E. Zhang, P.Ju, Z. Zhang, H. Yang, L.Tang, X.Hou, J. J. Wang, A novel multi-purpose Zn-MOF fluorescent sensor for 2, 4-dinitrophenylhydrazine, picric acid, La3+ and Ca2+: Synthesis, structure, selectivity, sensitivity and recyclability, Spectrochim. Acta Part A: Mol. Biomol. Spect., 222 (2019) 117207. https://doi.org/10.1016/j.saa.2019.117207
K. Ahmad, A. Mohammad, P. Mathur, S. M. Mobin, Preparation of SrTiO3 perovskite decorated rGO and electrochemical detection of nitroaromatics, Electrochim. Acta, 215 (2016) 435-446. https://doi.org/10.1016/j.electacta.2016.08.123
G. Zgherea, C. Stoian, S. Peretz, synthesis and physico-chemical characterization of 2.4-dinitrophenyl hidrazones derived from carbonyl compounds with some importance in the study of food quality, Annals of the University Dunarea de Jos of Galati Fascicle VI-Food Technol., 33 (2009) 83-89. https://ores.su/en/journals/annals-of-the-university-dunarea-de-jos-of-galati-fascicle-vi-food-technology
Y. Q. Lu, J. K. Jiang, W. D. Huang, Clinical features and treatment in patients with acute 2, 4-dinitrophenol poisoning, J. Zhejiang Uni. Sci. B, 12 (2011) 189-192. https://doi.org/10.1631/jzus.B1000265
M. A. Smith, L. M.Sayre, V. E. Anderson, P. L. Harris, M. F. Beal, N. Kowall, G. Perry, Cytochemical demonstration of oxidative damage in Alzheimer disease by immunochemical enhancement of the carbonyl reaction with 2, 4-dinitrophenylhydrazine, J. Histochem. Cytochem., 46 (1998) 731-735. http://www.jhc.org
F. Talebzadeh, S. Sobhanardakani, R. Zandipak, Effective adsorption of As (V) and V (V) ions from water samples using 2, 4-dinitrophenylhydrazine functionalized sodium dodecyl sulfate-coated magnetite nanoparticles, Sep. Sci. Technol., 52 (2017) 622-633. https://doi.org/10.1080/01496395.2016.1262873
S. Sobhanardakani, M. Ahmadi, R. Zandipak, Efficient removal of Cu(II) and Pb(II) heavy metal ions from water samples using 2,4-dinitrophenylhydrazine loaded sodium dodecyl sulfate-coated magnetite nanoparticles, J. Water Sup. Res. Technol. AQUA, 65 (2016) 361-372. https://doi.org/10.2166/aqua.2016.100
A. Afkhami, M. Saber-Tehrani, H. Bagheri, Simultaneous removal of heavy-metal ions in wastewater samples using nano-alumina modified with 2, 4-dinitrophenylhydrazine, J. Hazard. Mater., 181 (2010) 836-844. https://doi.org/10.1016/j.jhazmat.2010.05.089
S. Sobhanardakani, R. Zandipak, 2, 4-Dinitrophenylhydrazine functionalized sodium dodecyl sulfate-coated magnetite nanoparticles for effective removal of Cd(II) and Ni(II) ions from water samples, Environ. Monit. Assess., 187 (2015) 1-14. https://doi.org/10.1007/s10661-015-4635-y
I. Dalle-Donne, M. Carini, M. Orioli, , G. Vistoli, L. Regazzoni, G. Colombo, G. Aldini, Protein carbonylation: 2, 4-dinitrophenylhydrazine reacts with both aldehydes/ketones and sulfenic acids, Free Radical Biol. Med., 46 (2009) 1411-1419. https://doi.org/10.1016/j.freeradbiomed.2009.02.024
P. D. Neuenfeldt, B. B. Drawanz, G. M. Siqueira, C. R.Gomes, S. M. Wardell, A. F. Flores, W. Cunico, Efficient solvent-free synthesis of thiazolidin-4-ones from phenylhydrazine and 2, 4-dinitrophenylhydrazine, Tetrahedron Lett., 51 (2010) 3106-3108. https://doi.org/10.1016/j.tetlet.2010.04.026
J. Pilz, I. Meineke, C.H. Gleiter, Measurement of free and bound malondialdehyde in plasma by high-performance liquid chromatography as the 2, 4-dinitrophenylhydrazine derivative, J. Chromatogr. B Biomed. Sci. Appl., 742 (2000) 315-325. https://doi.org/10.1016/S0378-4347(00)00174-2 .
Y.L. Lin, P.Y. Wang, L.L. Hsieh, K.H. Ku, Y.T. Yeh, C.H. Wu, Determination of linear aliphatic aldehydes in heavy metal containing waters by high-performance liquid chromatography using 2, 4-dinitrophenylhydrazine derivatization, J. Chromatogr. A, 1216 (2009) 6377-6381. https://doi.org/10.1016/j.chroma.2009.07.018.
O. Korchazhkina, C. Exley, S.A. Spencer, Measurement by reversed-phase high-performance liquid chromatography of malondialdehyde in normal human urine following derivatisation with 2, 4-dinitrophenylhydrazine, J. Chromatogr. B, 794 (2003) 353-362. https://doi.org/10.1016/S1570-0232(03)00495-1.
S. Uchiyama, Y. Inaba, N. Kunugita, Derivatization of carbonyl compounds with 2, 4-dinitrophenylhydrazine and their subsequent determination by high-performance liquid chromatography, J. Chromatogr. B, 879 (2011) 1282-1289. https://doi.org/10.1016/j.jchromb.2010.09.028
R. Nawaz, T. Rasheed, T. Iqbal, M. Bilal, S. Majeed, Development of 2,4-dinitrophenylhydrazine-modified carbon paste electrode for highly sensitive electrochemical sensing of amino acids, Monatshefte für Chemie-Chemical Monthly, 151 (2020) 505-510. https://doi.org/10.1007/s00706-020-02580-y
F. Lipari, S. J. Swarin, 2, 4-Dinitrophenylhydrazine-coated Florisil sampling cartridges for the determination of formaldehyde in air, Environ. Sci. Technol., 19 (1985) 70-74. https://doi.org/10.1021/es00131a007
F. Soglia, M. Petracci, P. Ertbjerg, Novel DNPH-based method for determination of protein carbonylation in muscle and meat, Food Chem., 197 (2016) 670-675. https://doi.org/10.1016/j.foodchem.2015.11.038
T. Wang, X. Gao, J. Tong, L. Chen, Determination of formaldehyde in beer based on cloud point extraction using 2, 4-dinitrophenylhydrazine as derivative reagent, Food Chem., 131 (2012), 1577-1582. https://doi.org/10.1016/j.foodchem.2011.10.021
M. Al-Ani, L. U. Opara, D. Al-Bahri& N. Al-Rahbi, Spectrophotometric quantification of ascorbic acid contents of fruit and vegetables using the 2, 4-dinitrophenylhydrazine method, J. Food Agri. Environ., 5 (2007) 165. www.world-food.net
P.S. Praveen, B. Anupama, V. Jagathi, G.D. Rao, Spectrophotometric determination of Tolperisone using 2, 4-dinitrophenylhydrazine reagent, Int. J. Res. Pharm. Sci., 3 (2010) 317-320. www.ijrps.pharmascope.org
P. Nagaraja, A. K. Shrestha, Spectrophotometric method for the determination of drugs containing phenol group by using 2, 4-dinitrophenylhydrazine, E-J. Chem., 7 (2010) 395-402. https://doi.org/10.1155/2010/328061
H. Li, W. Goldberg, L. Verheyen, M. Foston, A method for the quantification of surface aldehyde content in cellulose nanocrystals using 2, 4-dinitrophenylhydrazine, SSRN J., (2022) 1-15. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4148789
R. M. Khaleel, D. H. Mohammed, (2020, November). Spectrophotometric determination of tetracycline hydrochloride using 2, 4–dinitrophenyl hydrazine as coupling reagent, J. Phys. Conf. Ser., 1664 (2020) 012084. https://doi.org/10.1088/1742-6596/1664/1/012084.
D. H. Mohammed, F. K. Omar, Spectrophotometric determination of catechol and resorcinol by oxidative coupling with 2, 4-dinitrophenyl hydrazine, Egypt. J. Chem., 64 (2021) 5061-5065. https://doi.org/10.21608/ejchem.2021.57506.3253
M. Sireesha, R.S. Chandan, B.M. Gurupadayya, A. Shravya, Spectrophotometric determination of Nateglinide using 2, 4-dinitrophenyl hydrazine and potassium ferricyanide in pharmaceutical dosage form, Der Pharma Chem., 3 (2011), 497-506. https://www.derpharmachemica.com
N. Rahman, S. Sameen, M. Kashif, Spectroscopic study on the interaction of haloperidol and 2, 4-dinitrophenylhydrazine and its application for the quantification in drug formulations, Anal. Chem. Lett., 6 (2016) 874-885. https://doi.org/10.1080/22297928.2016.1265898.
S. Behera, R. Behura, M. Mohanty, R. Dinda, P. Mohanty, A.K. Verma, B.R. Jali, (2020). Spectroscopic, cytotoxicity and molecular docking studies on the interaction between 2, 4-dinitrophenylhydrazine derived Schiff bases with bovine serum albumin, Sens. Int., 1 (2020) 100048. https://doi.org/10.1016/j.sintl.2020.100048
M. Kiamehr, B. Alipour, M. Nasrollahzadeh, S. M. Sajadi, Catalytic reduction of 2, 4‐dinitrophenylhydrazine by cuttlebone supported Pd NPs prepared using Conium maculatum leaf extract, IET Nanobiotechnol., 12 (2018) 217-222. https://doi.org/10.1049/iet-nbt.2017.0005
W. Boumya, M. Achak, M. Bakasse, M. A. El Mhammedi, Indirect determination of dopamine and paracetamol by electrochemical impedance spectroscopy using azo coupling reaction with oxidized 2, 4-dinitrophenylhydrazine (DNPH): Application in commercial tablets, J. Sci. Adv. Mater. Dev., 5 (2020) 218-223. https://doi.org/10.1016/j.jsamd.2020.04.003
M. P. Georgopoulou, C. V. Chrysikopoulos, Evaluation of carbon nanotubes and quartz sand for the removal of formaldehyde–(2, 4-dinitrophenylhydrazine) from aqueous solutions, Ind. Eng. Chem. Res., 57 (2018) 17003-17012. https://doi.org/10.1021/acs.iecr.8b03996
A. Mohammad, M. Ehtisham Khan, M. Hwan Cho, Sulfur-doped-graphitic-carbon nitride (S-g-C3N4) for low cost electrochemical sensing of hydrazine, J. Alloys Compd., 816 (2020) 152522. https://doi.org/10.1016/j.jallcom.2019.152522
I. Kolesnyk, J. Kujawa, H. Bubela, V. Konovalova, A. Burban, A. Cyganiuk, W. Kujawski, Photocatalytic properties of PVDF membranes modified with g-C3N4 in the process of Rhodamines decomposition, Sep. Purif. Technol., 250 (2020) 117231. https://doi.org/10.1016/j.seppur.2020.117231
H. Mirzaei, M. H. Ehsani, A. Shakeri, M. R.Ganjali, A. Badiei, Preparation and photocatalytic application of ternary Fe3O4/GQD/g-C3N4 heterostructure photocatalyst for RhB degradation, J. Pollut., 8 (2022) 779-791. https://doi.org/10.22059/POLL.2022.331685.1202
G. K. Jayaprakash, B. K. Swamy, S. Rajendrachari, S. C. Sharma, R. Flores-Moreno, Dual descriptor analysis of cetylpyridinium modified carbon paste electrodes for ascorbic acid sensing applications, J. Mol. Liq., 334 (2021) 116348. https://doi.org/10.1016/j.molliq.2021.116348.
K. I. Alabid, H. N. Nasser, Study of the behavior and determination of phenol based on modified carbon paste electrode with nickel oxide-nitrogen carbon quantum dots using cyclic voltammetry, Anal. Methods in Environ. Chem. J., 6 (2023) 58-68. https://doi.org/10.24200/amecj.v6.i01.227
K. I. Alabid, H. N. Nasser, An analytical method based on a modified carbon paste electrode by nanoparticles in optimal conditions for determining phenol in the liquid solutions and comparing it to high-performance liquid Chromatography, Anal. Methods in Environ. Chem. J., 6 (2023) 55-70. https://doi.org/10.24200/amecj.v6.i02.240
K. I. Alabid, H.N. Nasser, Synthesis and characterization of nickel oxide with nitrogen quantum carbon dots as nanoadsorbent (NiO-NCQD) nanocomposite. Int. J. Nano Dimens., 14 (2023) 227-237. https://doi.org/10.22034/IJND.2023.1984570.2217
W. Boumya, H. Hammani, F. Laghrib, S. Lahrich, A. Farahi, M. Achak, M. E.Mhammedi, (2017). Electrochemical study of 2, 4‐dinitrophenylhydrazine as derivatization reagent and aldehydes at carbon glassy electrode, Electroanal., 29 (2017) 1700-1711. https://doi.org/10.1002/elan.201700019
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________