Rapid extraction and separation of mercury in water and food samples based on micelles and azo-thiazoles complexation before determination by UV-Vis spectrophotometer
Volume 6, Issue 04, Pages 19-36, Dec 2023 *** Field: Analytical Food Chemistry
Abstract
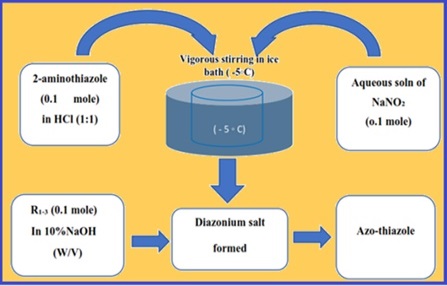
A simple and sensitive procedure has been established for analyzing mercury (II) ions spectrophotometrically in the presence of micellar medium using three azo-thiazoles complexing reagents: 2-amino-6-(thiazole-2-yldiazenyl)-3-pyridinol (C8H7N5OS), 8-hydroxy-7-(thiazole-2-yldiazenyl) quinoline-5-sulfonic acid (C12H8N4O4S2), and 1-hydroxy-4-(thiazole-2-yldiazenyl)-2-naphthoic acid (C14H9N3O3S). H1 NMR spectra validated the three azo thiazoles synthesized material. Tween 80 (polysorbate 80) and cetyltrimethylammonium bromide (C19H42BrN as molecular biology) are micellar mediums to enhance sensitivity. Absorbances were measured for Hg (II) complexation with R1, R2, and R3 at λmax of 617, 633, and 554 nm, respectively. The UV-Vis spectrophotometer showed calibration curves in the 0.2-15 mg L-1. The molar absorptivity, Sandell's sensitivity, detection, and quantification limits (LOD, LOQ) were determined. The interferences of various ions were investigated, and a statistical assessment of the results was performed. The methods have been applied for trace determination of mercury (II) in food and environmental water samples. For food samples, all samples were digested before complexation with the azo-thiazoles material at optimized pH before determination by UV-Vis spectrophotometry.
References
M. Salgarello, G. Visconti, L.B. Adesi, Interlocking circumareolar suture with undyed polyamide thread: a personal experience, Aesthetic Plast. Surg., 37 (2013) 1061-1062. https://doi.org/10.1007/s00266-013-0186-1
R. Gürkan, T. Çepken, H.I. Ulusoy, Surfactant-sensitized spectrophotometric determination of Hg (II) in water samples using 2-(2-thiazolylazo)-p-cresol as ligand and cetylpyridinium chloride as cationic surfactant, Turk. J. Chem., 36 (2012) 159-177. https://doi.org/10.3906/kim-1104-32
A. Ghaffari, Performance comparison of neural network training algorithms in modeling of bimodal drug delivery, Int. J. Pharm., 327 (2006) 126-138. https://doi.org/10.1016/j.ijpharm.2006.07.056
M. Saber Tehrani, F. Rastegar, A. Parchehbaf, M. Khatamian, Determination of Pb (II) and Cu (II) by electrothermal atomic absorption spectrometry after preconcentration by a Schiff base adsorbed on surfactant coated alumina, Chin. J. Chem., 24 (2006) 765-769. https://doi.org/10.1002/cjoc.200690145
M.S. Hosseini, H. Hashemi-Moghaddam, Sensitized extraction spectrophotometric determination of Hg (II) with dithizone after its flotation as ion-associate using iodide and ferroin, Talanta, 67 (2005) 555-559. https://doi.org/10.1016/j.talanta.2005.01.010
R.M. Blanco, M. Tagle Villanueva, J. Enrique Sánchez Urı́a, A. Sanz-Medel, Field sampling, preconcentration and determination of mercury species in river waters, Anal. Chim. Acta, 419 (2000) 137-144. https://doi.org/10.1016/S0003-2670(00)01002-3
A.N. Anthemidis, G. A. Zachariadis, C. E. Michos, J. A. Stratis, Time-based on-line preconcentration cold vapour generation procedure for ultra-trace mercury determination with inductively coupled plasma atomic emission spectrometry, Anal. Bioanal. Chem., 379 (2004) 764-769. https://doi.org/10.1007/s00216-004-2593-2
S. Rıo-Segade, C. Bendicho, Determination of total and inorganic mercury in biological and environmental samples with on-line oxidation coupled to flow injection-cold vapor atomic absorption spectrometry, Spectrochim. Acta Part B: Atom. Spect., 54 (1999)1129-1139. https://doi.org/10.1016/S0584-8547(99)00052-X
Q. Tu, J. Qvarnström, W. Frech, Determination of mercury species by capillary zone electrophoresis-inductively coupled plasma mass spectrometry: a comparison of two spray chamber–nebulizer combinations, Analyst, 125 (2000) 705-710. https://doi.org/10.1039/A908880F
M. Bacci, D. Magrini, M. Picollo, M. Vervat, A study of the blue colors used by Telemaco Signorini (1835–1901), J. Cult. Herit., 10 (2009) 275-280. https://doi.org/10.1016/j.culher.2008.05.006
V. Fernández-Pérez, L.E. Garcia-Ayuso, M. L. de Castro, Focused microwave Soxhlet device for rapid extraction of mercury, arsenic and selenium from coal prior to atomic fluorescence detection, Analyst, 125 (2000) 317-322. https://doi.org/10.1039/A905217H
S. Suresha, M. Fawaz Silwadi, A. Ahmed Syed, Sensitive and selective spectrophotometric determination of Hg (II), Ni (II), Cu (II) and Co (II) using iminodibenzyl and 3-chloroiminodibenzyl as new reagents and their applications to industrial effluents and soil samples, Int. J. Environ. Anal. Chem., 82 (2002) 275-289. https://doi.org/10.1080/03067310290024300
C.C. MagalhŃes, F. Krug, A.H. Fostier, Direct determimnation of mercury in sediments by atomic absorption spectrometry, J. Anal. At. Spectrom., 12 (1997) 1231-1234. https://doi.org/10.1039/A701870C
D. C. Nambiar, N. N. Patil, V.M. Shinde, Liquid-liquid extraction of mercury (II) with triphenylphosphine sulphide: Application to medicinal and environmental samples, Fresenius J. Anal. Chem., 360 (1998) 205-207. https://doi.org/10.1007/s002160050675
K.L. Cheng, K. Ueno, T. Imainura, Handbook of organic analytical reagents, CRC Press, 1982. https://library.unitech.ac.pg/cgi-bin/koha/opac-detail.pl?biblionumber=25868
H.Z. Mousavi, M.M. Eskandari, A.A. Miran-Beigi, Ultra-trace arsenic and mercury speciation and determination in blood samples by ionic liquid-based dispersive liquid-liquid microextraction combined wif flow injection-hydride generation/cold vapor atomic absorption spectroscopy, Chem. Papers, 69 (2015) 779-790. https://doi.org/10.1515/chempap-2015-0086
M. Osanloo, M. Ghazaghi, H. Hassani, Validation of a new and cost-effective method for mercury vapor removal based on silver nanoparticles coating on micro glassy balls, Atmos, Pollut. Res. 8 (2017) 359-365. https://doi.org/10.1016/j.apr.2016.10.004
A. Rouhollahi, Determination of mercury concentration in the air of dental clinics and the urines of their personnel with cold vapor atomic absorption spectrometry, Iran. J. Toxicol., 2 (2009), 287-291 http://ijt.arakmu.ac.ir/article-1-66-en.html
M. Osanloo, O. Qorban Dadrass, Using silver nano particles for sampling of toxic mercury vapors from industrial air sample, J. Health Safe. Work, 4 (2014), 21-30. http://jhsw.tums.ac.ir/article-1-5119-en.html
F. Golbabaei, A. Vahid, A. Faghihi Zarandi, A novel nano-palladium embedded on the mesoporous silica nanoparticles for mercury vapor removal from air by the gas field separation consolidation process, Appl. Nanosci., 12 (2022), 1667-1682. https://doi.org/10.1007/s13204-022-02366-0
F. Golbabaei, H. Hassani, F. Eftekhar, M.J. Kian, Occupational exposure to mercury: air exposure assessment and biological monitoring based on dispersive ionic liquid-liquid microextraction, Iran. J. Public Health, 43 (2014) 793-799. http://ijph.tums.ac.ir
M. Bagheri Hosseinabadi, N. Khanjani, M.D. Mobarake, Neuropsychological effects of long-term occupational exposure to mercury among chloralkali workers, Work, 66 (2020) 491-498. https://doi.org/ 10.3233/WOR-203194
F Golbabaei, A Ebrahimi, A Koohpaei, A Faghihi-Zarandi, Single-walled carbon nanotubes (SWCNTs), as a novel sorbent for determination of mercury in air, Global J. Health Sci., 8 (2016) 273-280. https://doi.org/10.5539/gjhs.v8n7p273
M. Habibnia, A. Rashidi, A.F. Zarandi, M.D. Mobarake, Simultaneously speciation of mercury in water, human blood and food samples based on pyrrolic and pyridinic nitrogen doped porous, graphene nanostructure, Food Chem., 403 (2023) 134394 2023. https://doi.org/10.1016/j.foodchem.2022.134394
F. Golbabai, A. Ebrahimi, Performance comparison survey of multi-walled and single-walled carbon nanotubes for adsorption and desorption of Mercury Vapors in the air, Iran Occup. Health, 10 (2013), 21-31. https://espace.library.uq.edu.au/view/UQ:9d03f02
A. I. Vogel, Ά textbook of quantitative inorganic analysis, Longman, London, 1978. https://archive.org/details/vogelstextbookof0000voge
A. B. Fowler, R. K. Zalups, Mercury, Chapter 22, Handbook on the Toxicology of Metals, Academic press, fifth Edition, Volume II (2022) 539-599. https://doi.org/10.1016/B978-0-12-822946-0.00020-9
J.H. Yoe, A. Jones, Colorimetric determination of iron with disodium-1, 2-dihydroxybenzene-3, 5-disulfonate, Am. Chem. Soc., 16 (1944)111-115. https://doi.org/10.1021/i560126a015
A. Keramat, R. Zare-Dorabei, Ultrasound-assisted dispersive magnetic solid phase extraction for preconcentration and determination of trace amount of Hg (II) ions from food samples and aqueous solution by magnetic graphene oxide (Fe3O4@ GO/2-PTSC): Central composite design optimization, Ultrason. Sonochem. 38 (2017) 421-429. https://doi.org/10.1016/j.ultsonch.2017.03.039
M.H. Mashhadizadeh, Solid phase extraction of trace amounts of silver, cadmium, copper, mercury, and lead in various food samples based on ethylene glycol bis-mercaptoacetate modified 3-(trimethoxysilyl)-1-propanethiol coated Fe3O4 nanoparticles, Food Chem.,151 (2014) 300-305. https://doi.org/10.1016/j.foodchem.2013.11.082
M.A. Kassem, I. I. Althagafi, Sensitive spectrofluorimetric study of the interaction between Europium (III) and 1, 2-Phenylenebis (azan-1-yl-1-ylidene) bis (methan-1-yl-1-ylidene) diphenol Schiff Base, J. Fluorescence, 26 (2016) 2087-2093. https://doi.org/10.1007/s10895-016-1903-3
Y.G. Yin, Dithizone-functionalized solid phase extraction–displacement elution-high performance liquid chromatography–inductively coupled plasma mass spectrometry for mercury speciation in water samples, Talanta, 81 (2010) 1788-1792. https://doi.org/10.1016/j.talanta.2010.03.039
L. Li, Transformation of cefazolin during chlorination process: products, mechanism and genotoxicity assessment, J. Hazard. Mater., 262 (2013) 48-54. https://doi.org/10.1016/j.jhazmat.2013.08.029
A. Niazi, T. Momeni-Isfahani, Spectrophotometric determination of mercury in water samples after cloud point extraction using nonionic surfactant Triton X-114, J. Hazard. Mater., 165 (2009) 1200-1203. https://doi.org/10.1016/j.jhazmat.2008.09.091
A. Afkhami, T. Madrakian, H. Siampour, Flame atomic absorption spectrometric determination of trace quantities of cadmium in water samples after cloud point extraction in Triton X-114 without added chelating agents, J. Hazard. Mater., J. Hazard. Mater., 138 (2006) 269-272. https://doi.org/10.1016/j.jhazmat.2006.03.073
M. Pandurangappa, K.S. Kumar, Micellar mediated trace level mercury quantification through the rhodamine B hydrazide spirolactam ring opening process, Anal. Methods, 3 (2011) 715-723. https://doi.org/10.1039/C0AY00693A
M. Garrido, M.S. Di Nezio, A.G. Lista, M. Palomeque, B.F. Band, Cloud-point extraction/preconcentration on-line flow injection method for mercury determination. Anal. Chim. Acta, 502 (2004) 173-177. https://doi.org/10.1016/j.aca.2003.09.070
H. I. Ulusoy, R. Gürkan, S. Ulusoy, Cloud point extraction and spectrophotometric determination of mercury: species at trace levels in environmental samples, Talanta, 88 (2012) 516-523. https://doi.org/10.1016/j.talanta.2011.11.026
M. Arjomandi, A review: analytical methods for heavy metals determination in environment and human samples, Anal. Methods in Environ. Chem. J. 2 (2019) 97-126. https://doi.org/10.24200/amecj.v2.i03.73
H.B. Singh, B. Kumar, R.L. Sharma, M. Katyal, Direct spectrophotometric determination of trace amounts of mercury (II) in aqueous media as its dithizonate complex in the presence of a neutral surfactant, Analyst, 114 (1989) 853-855. https://doi.org/10.1039/AN9891400853
Copyright (c) 2023 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________