Speciation and removal of selenium (IV, VI) from water and wastewaters based on dried activated sludge before determination by flame atomic absorption spectrometry
Volume 4, Issue 01, Pages 36-45, Mar 2021 *** Field: Analytical Environmental Chemistry
Abstract
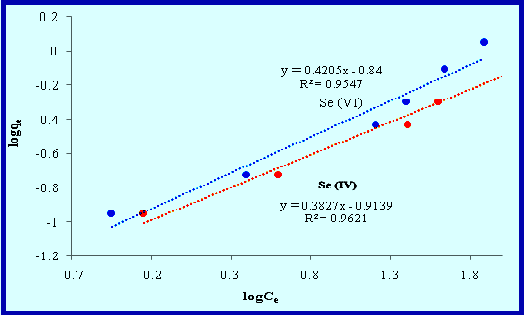
In recent decades, large amount of pollutants enter to the environment due to development of technology. Therefore it is necessary to use ecofriendly sorbent to eliminate pollutants. In this research, an dried activated sludge (DAS) was used to decrease Se(IV) pollutant and the effect of operating parameters such as solution pH, the amount of biosorbent, contact time, temperature and initial concentration of selenium were studied. Kinetic data was adjusted to the Langmuir and Freundlich kinetic equations. It was resulted that the Freundlich equation with a correlation coefficient of 0.9956 has the Best match to tetravalent selenium biosorption on DAS. The FT-IR results showed that the biosorption mechanism of selenium tetravalent metal ion on DAS is because of the existence of functional groups on the DAS surface can interact with Se(IV) ions. This study aims to investigate the biosorption capacity of the DAS for tetravalent selenium ions in aqueous solutions.
References
M. Kashiwa, S. Nishimoto, K. Takahashi, M. Ike, M. Fujita, Factors affecting soluble selenium removal by a selenite reduciing bacterium Bacillus sp. SF-1, J. Biosci. Bioeng., 89 (2000) 528-533.
M. Tuzen, A. Sari, Biosorption of selenium from aqueous solution by green alga (Cladophorahutchinsiae) biomass: Equilibrium, thermodynamic and kinetic studies, Chem. Eng. J., 158 (2010) 200-206.
M. Rovira, J. Gimienez, M. Martiinez, X. Marttinez-Lladio, J. Pablo, V. Martii, L. Duro, Sorption of selenium(IV) and selenium(VI) onto natural iron oxides: goethite and hematite, J. Hazard. Mater., 150 (2008) 279-284.
F.M. Fordyce, selenium deficiency and toxicity in the environment, Essentials of Medical Geology, Elsevier Academic Press, Amsterdam, Holland, 2005, pp.373-416.
K. Nettem, A.S. Almusallam, Equilibrium, kinetic, and themodynamic studies on the biosorption of selenium (IV) ions onto Ganoderma Lucidum Biomass, Sep. Sci. Tech., 48 (2013) 2293-2301.
H. Robberecht, R.V. Grieken, Selenium in environmental water: determination, speciation and concentration levels, Talanta, 29 (1982) 823-844.
J. Lessa, A. Araujo, G. Silva, L. Guilherme, G. Lopes, Adsorption-desorption reactions of selenium (VI) in tropical cultivated and uncultivated soils under Cerrado biome, Chemosphere, 164 (2016) 271-277.
S. Santos, G. Ungureanu, R. Boaventura, C. Botelho, Selenium contaminated waters: an overview of analytical methods, treatment options and recent advances in sorption methods, Sci. Total Environ., 521-522 (2015) 246-260.
E.I. El-Shafey, Sorption of Cd(II) and Se(IV) from aqueous solution using modified rice husk, J. Hazard. Matter., 147 (2007) 546- 555.
F.Sahin, M. Volkan, A.G. Howard, O.Y. Ataman, Selective preconcentration of selenite from aqueous samples using mercapto-silica, Talanta, 60 (2003) 1003-1009.
G. Kallis, D. Butler, The EU water framework directive: measures and implications, Water Policy, 3 (2001) 125- 142.
O.Y. Bakather, A. Kayvani fard, Ihsanullah, M. Khraisheh, M.S. Nasser, M.A. Atieh, Enhanced adsorption of selenium ions from aqueous solution using iron oxide impregnated carbon nanotubes, Bioinorg. Chem. Appl., 2017 (2017) 1-12.
M. Kieliszek, S. Blazejak, K. Piwowarek, K. Brzezicka, Equilibrium modeling of selenium binding from aqueous solutions by Candida atilis ATCC 9950 yeasts, Biotech. 8 (2018) 388: doi: 10.1007/s13205-018-1415-8.
F.A. Bertolino, A.A.J. Torriero, E. Salinas, R. Olsina, L.D. Martinez. J. Raba, Speciation analysis of selenium in natural water using square-wave voltammetry after preconcentration on activated carbon, Anal. Chim. Acta 572 (2006) 32-38.
S. Dev, A. Khamkhash, T. Ghosh, S. Aggarwal, Adsorptive removal of Se(IV) by Citrus peels: Effect of adsorbent entrapment in calcium alginate beads, ACS Omega, 5 (2020) 17215-17222.
R.H.S.F. Vieira, B. Volesky, Biosorption: a solution to pollution, Int. Microbiol., 3 (2000) 17-24.
D.A. Roberts, N.A. Paul, S.A. Dworjanyn, Y. Hu, M.I. Bird, R. de Nys, Gracilaria waste biomass (sampah rumput laut) as a bioresource for selenium biosorption, J. Appl. Phycol., 27 (2015) 611-620.
S.H. Hasan, D. Ranjan, Agro-industerial waste: a low-cost option for the biosorptive remediation 0f selenium anions, Ind. Eng. Chem. Res., 49 (2010) 8927-8934.
S. Beeram, A. Morris, C.J. Hardway, J.C. Richert, J. Sneddon, Studies of whole crawfish shells for the removal of chromium, lead and selenium ions in solution, Instrum. Sci. Technol., 40 (2012) 618-639.
H. Khakpour, H. Younesi, M. Mohammadhosseini, Two-stage biosorption of selenium from aqueous solution using dried biomass of the baker’s yeast Saccharomyces cerevisiae, J. Environ. Chem. Eng., 2 (2014) 532-542.
H. Zare, H. Heydarzadeh, M. Rahimnejad, A. Tardast, M. Seyfi, S.M. Peyghambarzade, Dried activated sludge as an appropriate biosorbent for removal of copper (II) ions, Arab. J. Chem., 8 (2015) 858-864.
L. Bennamoun, P. Arlabosse, A. Leonard. Review on fundamental aspect of application of drying process to wastewater sludge, Renew. Sust. Energ. Rev., 28 (2013) 29-43.
H. Shirkhanloo, F. Golbabaei, H. Hassani, F. Eftekhar, M.J. Kian, Occupational exposure to mercury: air exposure assessment and biological monitoring based on dispersive ionic liquid-liquid microextraction, Iran. J. Public Health, 43 (2014) 793.
S. Golkhah, H. Zavvar Mousavi, H. Shirkhanloo, A. Khaligh, Removal of Pb (II) and Cu (II) Ions from Aqueous Solutions by Cadmium Sulfide Nanoparticles, Int. J. Nanosci. Nanotechnol., 13 (2017) 105-117.
H. Shirkhanloo, A. Khaligh, H.Z. Mousavi, A. Rashidi, Graphene oxide-packed micro-column solid-phase extraction combined with flame atomic absorption spectrometry for determination of lead (II) and nickel (II) in water samples, Int. J. Environ. Anal. Chem., 95 (2015) 16-32.
M. Arjomandi, H. Shirkhanloo, A review: Analytical methods for heavy metals determination in environment and human samples, Anal. Methods Environ. Chem. J., 2 (2019) 97-126.
H. Shirkhanloo, S. A. H. Mirzahosseini, N. Shirkhanloo, The evaluation and determination of heavy metals pollution in edible vegetables, water and soil in the south of Tehran province by GIS, Arch. Environ. Protec., 41 (2015) 64-74.
M. Duc, G. Lefevre, M. Fedoroff, Sorption of selenite ions on hematite, J. Colloid Interface Sci., 298 (2006) 556-563.
T. Nishimura, H. Hashimoto, M. Nakayama, Removal of selenium(VI) from aqueous solution with poly-amine type weakly basic ion exchange resin, Sep. Sci. Technol., 42 (2007) 3155-3167.
I. Langmuir, The adsorption of gases on plane surfaces of glass, mica and platinum, J. Am. Chem. Soc., 40 (1918) 1361–1403.
H. Freundlich, Adsorption in solution. J. Phys. Chem. 57 (1906) 384–410.
Copyright (c) 2021 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________