Adsorption of nitrate from aqueous solution with ZSM-5/Fe nanosorbent based on optimizing of the isotherms conditions before determination by UV-Vis Spectrophotometry
Volume 4, Issue 04, Pages 49-63, Dec 2021 *** Field: Nano Spectrophotometry
Abstract
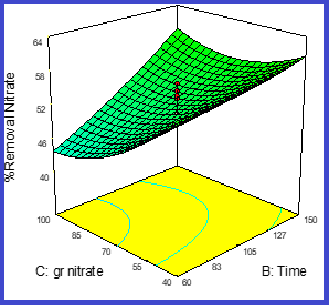
The life-threatening nature of high nitrate concentrations in various water resources motivated the present study to investigate the nitrate adsorption by ZSM-5 nanozeolite and the feasibility of increasing nitrate removal efficiency using iron-doped ZSM-5 (ZSM-5/Fe) nanosorbent. Energy dispersive X-ray diffraction analysis was employed to determine the physical properties of the adsorbent and the presence of iron particles in the nanosorbent structure and BET analysis to measure the specific surface area of the nanosorbent. The optimal adsorption conditions were determined first by modeling the central composite design (CCD) using Design Expert.7 software based on four influential factors . Then, the isotherms of nitrate adsorption under optimized conditions were investigated using the degree of fit of experimental data with Langmuir and Freundlich models for mathematical modeling of the nitrate adsorption process. Based on the test design results, the highest nitrate removal efficiency (%93.1) was reported at the contact time of 150 min, pH value of 3, adsorbent dosage of 4 g/l and initial concentration of 40 mg/l. Analysis of adsorption isotherms also confirmed the greater fit of the experimental data with the Freundlich equation, so that the correction factor of the Freundlich equation was greater than the Langmuir equation.
References
D.W. Cho, C.M. Chon, B.H. Jeon, Y. Kim, M.A. Khan, H. Song, The role of clay minerals in the reduction of nitrate in groundwater by zero-valent iron, Chemosphere, 81 (2010) 611-616. https://doi.org/j.chemosphere.2010.08.005
P. Mishra, R. Patel, Use of agricultural waste for the removal of nitrate-nitrogen from aqueous medium, J. Environ. Manage., 90 (2009) 519-22. https://doi.org/10.1016/j.jenvman.2007.12.003
M. Nikaeen, S. Naseri, Evalution of metallic iron (Fe0) application to remediate nitrate contaminated water, Water Wastewater, 17 (2007) 15-21. http://www.wwjournal.ir/article_2222.html?lang=en
S. Shadkam, F. Ludwig, P. van Oel, C. Kirmit, P. Kabat, Impacts of climate change and water resources development on the declining inflow into Iran's Urmia Lake, J. Great Lakes Res., 42 (2016) 942-952. https://doi.org/10.1016/j.jglr.2016.07.033.
Eslami A, Ghadimi M. Study of five years nitrite and nitrate content trends of Zanjan groundwater resources using GIS from 2006 to 2010, J. Health Field, 1 (2013) 30–6. http://journals.sbmu.ac.ir/jhf
R. Fouladi Fard, M. J. M. Abadi, M. R. Hosseini, Survey the nitrate concentration in drinking water distribution systems of Kashan county, Iran, J. Saf. Environ. Health Res., 1 (2016) 36–39. http://doi.org/ 10.22053/jsehr.2016.33387
M. Dehghani, E. Haidari, S. Shahsavani, N. Shamsedini, Removal of nitrate in the aqueous phase using granular ferric hydroxide, Jundishapur J. Health Sci., 7(2015) e26419. http://doi.org/ 10.5812/jjhs.7(2)2015.26419
J. Rodríguez-Maroto, F. García-Herruzo, A. García-Rubio, C. Gómez-Lahoz, C.Vereda-Alonso, Kinetics of the chemical reduction of nitrate by zero-valent iron, Chemosphere, 74 (2009) 804-809. http://doi.org/10.1016/jchemosphere.2008.10.020
I.F. Cheng, R. Muftikian, Q. Fernando, N. Korte, Reduction of nitrate to ammonia by zero valent iron, Chemosphere, 35 (1997) 2689–2695. http://doi.org/10.1016/S0045-6535(97)00275-0
Y.H. Huang, T.C. Zhang, Effects of low pH on nitrate reduction by iron powder, Water Res., 38 (2004) 2631–2642. https://doi.org/10.1016/j.watres.2004.03.015
Y.M. Chen, C.W. Li, S.S. Chen, Fluidized zero valent iron bed reactor for nitrate removal, Chemosphere, 59 (2005) 753–759. http://doi.org/10.1016/chemosphere.2004.11.020
Y.H. Liou, S.L. Lo, C.J. Lin, C.Y. Hu, W.H. Kuan, S.C. Weng, Methods for accelerating nitrate reduction using zerovalent iron at near-neutral pH: effects of H2-reducing pretreatment and copper deposition, Environ. Sci. Technol., 39 (2005) 9643–9648. http://doi.org/10.1021/es048038phttps://doi.org/
S.C. Ahn, S.Y. Oh, D.K. Cha, Enhanced reduction of nitrate by zero-valent iron at elevated temperatures, J. Hazard. Mater., 156 (2008) 17–22. http://doi.org/10.1016/j.jhazmat.2007.11.04
M. Dore, Ph. Simon, A. Deguin, J. Victot, Removal of nitrate in drinking water by ion exchange-impact on the chemical quality of treated water, Water Res., 20 (1986) 221–232. http://dx.doi.org/10.1016/0043-1354(86)90012-6
S. Samatya, N. Kabay, U. Yuksel, M. Arda, M. Yuksel, Removal of nitrate from aqueous solution by nitrate selective ion exchange resins, Reac. Funct. Polym., 66 (2006) 1206–1214. http://doi.org/10.1016/j.reactfunctpolym.2006.03.009
M. Chabani, A. Amrane, A. Bensmaili, Kinetic modelling of the adsorption of nitrates by ion exchange resin, Chem. Eng. J., 125 (2006) 111–117. http://doi.org/10.1016/j.cej.2006.08.014
J.J. Schoeman, A. Steyn, Nitrate removal with reverse osmosis in a rural area in South Africa, Desalination, 155 (2003)15–26. http://doi.org/10.1016/S0011-9164(03)00235-2
M.I.M. Soares, Biological denitrification of groundwater, Water Air Soil Pollut.,123 (2000) 183–193. https://doi.org/10.1023/A:1005242600186
A. Bhatnagar, M. Sillanpää, A review of emerging adsorbents for nitrate removal from water, Chem. Eng. J., 168 (2011) 493–504. http://doi.org/10.1016/j.cej.2011.01.103
E.Y. Emori, F.H. Hirashima, C.H. Zandonai, C.A. Ortiz-Bravo, N.R.C. Fernandes-Machado, M.H.N. Olsen-Scaliante, Catalytic cracking of soybean oil using ZSM5 zeolite. Catal. Today, 279 (2017)168–176. http://doi.org/10.1016/J.CATTOD.2016.05.052.
Q. Zhang. G. Liu. L. Wang. X. Zhang, G. Li, Controllable decomposition of methanol for active fuel cooling technology, Energey Fuels, 28 (2014) 4431–4439. http://doi.org/10.1021/ef500668q
W. Li, G. Li, C. Jin, X. Liu, J. Wang, One-step synthesis of nanorod-aggregated functional hierarchical iron-containing MFI zeolite microspheres. J. Mater. Chem. A, 3 (2015) 14786–14793. http://doi.org/10.1039/C5TA02662H
T.J. Pinnavaia, Intercalated clay catalysts, Science 220 (1983) 365–371. http://doi.org/10.1126/science.220.4595.365
K.G. Bhattacharyya, S.S. Gupta, Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review, Adv. Colloid Interface Sci.,140 (2008) 114–131. http://doi.org/10.1016/j.cis.2007.12.008
S. Wang, Y. Peng, Natural zeolites as effective adsorbents in water and wastewater treatment, Chem. Eng. J., 156 (2010) 11–24. https://doi.org/10.1016/j.cej.2009.10.029
B. Kamarehie, E. Aghaali, S.A. Musavi, S.Y. Hashemi, A. Jafari, Nitrate removal from aqueous solutions using granular activated carbon modified with Iron nanoparticles, Int. J. Eng. Transactions A, 31 (2018) 554-563. https://doi.org/10.5829/ije.2018.31.04a.06
M. Mazarji, B. Aminzadeh, M. Baghdadi, A. Bhatnagar, Removal of nitrate from aqueous solution using modified granular activated carbon, J. Mol. Liq., 233 (2017) 139-148. https://doi.org/10.1016/j.molliq.2017.03.004
L. Divband Hafshejani, A. Hooshmand, A.A. Naseri, A.S. Mohammadi, F. Abbasi, A. Bhatnagar, Removal of nitrate from aqueous solution by modified sugarcanebagasse biochar, Ecol. Eng., 95 (2016) 101–111. http://dx.doi.org/10.1016/j.ecoleng.2016.06.035
T. Meftah, M. M. Zerafat, Nitrate removal from drinking water using organo-silane modified natural nano-zeolite, Int. J. Nanosci. Nanotechnol.,12 (2016) 223-232. http://www.ijnnonline.net/article_22931.html
V. Alimohammadi, M. Sedighi, E. Jabbari, Response surface modeling and optimization of nitrate removal from aqueous solutions using magnetic multi-walled carbon nanotubes, J. Environ. Chem. Eng., 4 (2016) 4525–4535. http://dx.doi.org/10.1016/j.jece.2016.10.017
A. Azari, A.H. Mahvi, S. Naseri, R. Rezaei Kalantary, M. Saberi, Nitrate removal from aqueous solution by using nodified clinoptilolite zeolite, research center for environmental pollutants, Arch. Hyg. Sci., 3 (2014) 21-29. http://jhygiene.muq.ac.ir
S. Sepehri, M. Heidarpour, J. Abedi, Nitrate removal from aqueous solution using natural zeolite-supported zero-valent Iron nanoparticles, Soil Water Res., 9 (2014): 224–232. https://doi.org/10.17221/11/2014-SWR
E. Fataei, A. Seyyed Sharifi, H. Hasan Pour Kourandeh, A.S. Sharifi, S.T.S. Safavyan, Nitrate removal from drinking water in laboratory-scale using iron and sand nanoparticles, Int. J. Biosci.,10 (2013) 256-261. http://dx.doi.org/10.12692/ijb/3.10.256-261
A. Bhatnagar, E. Kumarb, M. Sillanpää, Nitrate removal from water by nano-alumina: characterization and sorption studies, Chem. Eng. J., 163 (2010) 317–323. https//doi/10.1016/j.cej.2010.08.008
R.M. McKeown, C. Scully, T. Mahony, G. Collins, V. O'Flaherty, Long-term (1243 days), low-temperature (4-15C), anaerobic biotreatment of acidified wastewaters,bioprocess performance and physiological characteristics, Water Res., 43 (2009) 1611-1620. http://doi.org/10.1016/j.watres.2009.01.015
C. Scully, G. Collins, V. O'Flaherty, Anaerobic biological treatment of phenol at 9.5-15 C in an expanded granular sludge bed (EGSB)-based bioreactor, Water Res., 40 (2006) 3737-3744. http://doi.org/10.1016/j.watres.2006.08.023
J. Lopez, V.M. Monsalvo, D. Puyol, A.F. Mohedano, J.J. Rodriguez, Low temperature anaerobic treatment of low-strength pentachlorophenol-bearing wastewater. Bioresour.Technol.,140 (2013) 349-356. http://doi.org/10.1016/j.biortech.2013.04.049
W.M. Bandara, H. Satoh, M. Sasakawa, Y. Nakahara, M. Takahashi, S. Okabe, Removal of residual dissolved methane gas in an upflow anaerobic sludge blanket reactor treating low-strength wastewater at low temperature with degassing membrane, Water Res.,45 (2011) 3533-3540. http://doi.org/10.1016/j.watres.2011.04.030
A.J.M. Stams, C.M. Plugge, Electron transfer in syntrophic communities of anaerobic bacteria and archaea, Nat. Rev. Microbiol., 7 (2009) 568-577. http://doi.org/10.038/nrmicro2166
T. Tian, S. Qiao, C. Yu, Y. Yang, J. Zhou, Low-temperature anaerobic digestion enhanced by bioelectrochemical systems equipped with graphene/PPy and MnO2 nanoparticles/PPy-modified electrodes, Chemosphere,218 (2019) 119-127(2019). http://doi.org/10.1016/j.chemosphere.2018.11.001
SIP References
SIP41- K. Glissmann, K.J. Chin, P.Casper, R. Conrad, Methanogenic pathway and archaeal community structure in the sediment of eutrophic Lake Dagow: effect of temperature. Microb. Ecol., 48 (2004) 389-399. http://www.jstor.org/stable/25153121
SIP42- O.Tkach, T. Sangeetha, S. Maria, A. Wang, Performance of low temperature Microbial Fuel Cells (MFCs) catalyzed by mixed bacterial consortia, J. Environ. Sci., 52 (2017) 284-292. http://doi.org/10.1016/j.jes.2016.11.006
SIP43- B.W. Chieng, N.A. Ibrahim, W.M.Z. Wan Yunus, Optimization of tensile strength of poly(Lactic Acid)/graphene nanocomposites using response surface methodology, Polymer-Plastics Technol. Eng., 51 (2012) 791–799. http://doi.org/10.1080/03602559.2012.663043
SIP44- B. Zhang, J. Zhang, Q. Yang, C. Feng, Y. Zhu, Z. Ye, J. Ni, Investigation and optimization of the novel UASB–MFC integrated system for sulfate removal and bioelectricity generation using the response surface methodology (RSM), Bioresour. Technol., 124 (2012),1–7. http://doi.org/10.1016/j.biortech.2012.08.045
SIP45- A.A. Zamani, R. Shokri, M.R. Yaftian, A.H. Parizanganeh, Adsorption of lead, zinc and cadmium ions from contaminated water onto Peganum harmala seeds as biosorbent, Int. J. Environ. Sci. Technol., 10 (2013) 93–102. http://doi.org/10.1007/s13762-012-0107-x.
SIP46- M.V. Dinu, E.S. Dragan, Evaluation of Cu2+,Co2+ and Ni2+ ions removal from aqueous solution using a novel chitosan/clinoptilolite composite: Kinetics and isotherms, Chem. Eng. J., 160 (2010) 157–163. http://doi.org/10.1016/j.cej.2010.03.029
SIP47- D. Humelnicu, M.V. Dinu, E.S.A. Dragan, Adsorption characteristics of UO22+ and Th4+ ions from simulated radioactive solutions onto chitosan/clinoptilolite sorbents, J. Hazard. Mater., 185 (2011) 447–455. http://doi.org/10.1016/j.jhazmat.2010.09.053
Copyright (c) 2021 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________