Separation and determination of lithium and manganese ions in healthy humans and multiple sclerosis patients based on Nano graphene oxide by Ultrasound assisted-dispersive -micro solid-phase extraction
Volume 4, Issue 04, Pages 20-35, Dec 2021 *** Field: Bioanalytical Chemistry
Abstract
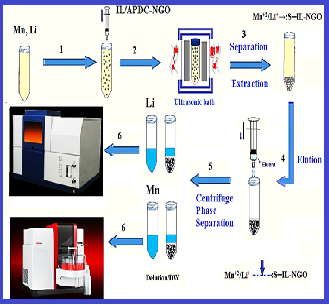
Lithium regulates the concentration of nitric oxide in the human body and a high dose of nitric oxide causes multiple sclerosis (MS). Also, the amount of manganese in the cerebrospinal fluid alters the metabolic reactions associated with MS. In this study, the mixture of the ammonium pyrrolidine dithiocarbonate (APDC), the hydrophobic ionic liquid [HMIM][PF6] and acetone coated on the surface of graphene oxide nanoparticles (GONPs) and used for separation Li and Mn in human samples by ultrasound assisted-dispersive-ionic liquid-micro-solid phase extraction technique (USA-DIL-μ-SPE) at pH 6.0. After extraction and back-extraction, the amount of lithium and manganese in the blood, serum and urine samples was determined by the flame and the graphite furnace atomic absorption spectroscopy (F-AAS, GF-AAS), respectively. By optimizing parameters, the LOD, Linear ranges (LR) and preconcentration factor (PF) for Li and Mn ions were obtained (0.03 mg L-1, 0.25 μg L-1), (0.1-0.4 mg L-1, 0.08-1.5 μg L-1) and 10, respectively (%RSD<5). The capacity adsorption of APDC/IL/GONPs and GONPs was achieved (148.5 mg g-1, 122.3 mg g-1) and (41.3 mg g-1, 33.7 mg g-1) for Li and Mn ions in a static system, respectively. This method was successfully validated by spiking samples and certified reference materials (CRM).
References
V.L. Feigin, A.A. Abajobir, K.H.Abate, F. Abd-Allah, A.M. Abdulle, S.F. Abera, Global regional and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of disease study 2015, Lancet Neurol.,16 (2017) 877-897. https://doi.org/10.1016/S1474-4422(17)30299-5
M.D. Napier, Heavy metals, organic solvents, and multiple sclerosis: an exploratory look at gene-environment interactions, Arch. Environ. Occup. Health, 71 (2016) 26-34. https://doi.org/10.1080/19338244.2014.937381
A. Karimi, K. Bahrampour, M.A.M. Moghaddam, G. Asadikaram, G. Ebrahimi, M. Torkzadeh-Mahani, Evaluation of lithium serum level in multiple sclerosis patients: A neuroprotective element, Mult. Scler. Relat. Disord., 17 (2017) 244-48. https://doi.org/10.1016/j.msard.2017.08.019
D.F.D. Mota, B. D. Leverson, J. L. Goossens, Lithium in medicine: mechanisms of action, Met. Ions Life Sci., 16 (2016) 557. https://doi.org/10.1007/978-3-319-21756-7_15
C. Garza-Lombó, Y. Posadas, L. Quintanar, M. E. Gonsebatt, R. Franco, Neurotoxicity linked to dysfunctional metal ion homeostasis and xenobiotic metal exposure: redox signaling and oxidative stress, Antioxid. Redox Signal, 28 (2018) 1669-1703. https://doi.org/10.1089/ars.2017.7272
P. Chen, S. Chakraborty, S. Mukhopadhyay, E. Lee, M. M.B. Paoliello, A. B. Bowman, M. Aschner, Manganese homeostasis in the nervous system, J. Neurochem., 134 (2015) 601-610. https://doi.org/10.1111/jnc.13170
R. Nicholas, R. Waqar, Multiple sclerosis, Am. Fam. Physician, 87 (2013) 712-714. https://pubmed.ncbi.nlm.nih.gov/23939450/
A. Winckelmann, D. Morcillo, S. Richter, S. Recknagel, J. Riedel, J. Vogl, U. Panne, C. Abad, Determination of lithium in human serum by isotope dilution atomic absorption spectrometry, ChemRxiv, (2021). https://doi.org/10.26434/chemrxiv.14130080.v1
M. Aliasgharpour, H. Hagani, Evaluation of lithium determination in three analyzers: flame emission, FAAS and ion-selective electrode, Am. J. Med., 1 (2009) 244. https://dx.doi.org/10.4297%2Fnajms.2009.5244
M. Aliomrani, M. A. Sahraian, H. Shirkhanloo, M. Sharifzadeh, M. R. Khoshayand, M. H. Ghahremani, Blood concentrations of cadmium and lead in multiple sclerosis patients from Iran, Iran. J. Pharm. Sci., 15 (2016) 825. https://www.ncbi.nlm.nih.gov/pubmed/28243279
M. de Oliveira, T.M. Gianeti, F.C. da Rocha, P.N. Lisboa-Filho, M. Piacenti-Silva, A preliminary study of the concentration of metallic elements in the blood of patients with multiple sclerosis as measured by ICP-MS, Sci. Rep., 10 (2020) 13112. https://doi.org/10.1038/s41598-020-69979-9
U. Kramer, M. Kress, H. Reinauer, M. Spannagl, P. Kaiser, Candidate reference measurement procedures for chloride, potassium, sodium, calcium, magnesium, and lithium by inductively coupled plasma (isotope dilution) sector field mass spectrometry (ICP-(ID) SFMS) in serum, Clin. Lab., 59 (2013) 1017-1029. https://doi.org/10.7754/clin.lab.2012.120902
J. Lin, Y. Liu, Z. Hu, L. Yang, K. Chen, H. Chen, K. Zong, S. Gao, Accurate determination of lithium isotope ratios by MC-ICP-MS without strict matrix-matching by using a novel washing method, J. Anal. At. Spectrom., 31 (2016) 390-397. https://doi.org/10.1039/C5JA00231A
A. Winckelmann, S. Nowak, S. Richter, S. Recknagel, J. Riedel, J. Vogl, U. Panne, C. Abad, High-Resolution atomic absorption apectrometry aombined withmachine learning data processing for isotope amount ratio analysis of lithium, ChemRxiv, (2021). https://doi.org/10.26434/chemrxiv.13583024.v1
E. Marguí, R. Dalipi, E. Sangiorgi, M. B. Štefan, K. Sladonja, V. Rogga, J. Jablan, Determination of essential elements (Mn, Fe, Cu and Zn) in herbal teas by TXRF, FAAS and ICP‐OES, Xray Spectrom., (2021)1-10. https://doi.org/10.1002/xrs.3241
N. Solovyev, M. Vinceti, P. Grill, J. Mandrioli, B. Michalke, Redox speciation of iron, manganese, and copper in cerebrospinal fluid by strong cation exchange hromatography–sector field inductively coupled plasma mass spectrometry, Anal. Chim. Acta, 973 (2017) 25-33. https://doi.org/10.1016/j.aca.2017.03.040
H. Shirkhanloo, M. Ghazaghi, M. M. Eskandari, Cloud point assisted dispersive ionic liquid-liquid microextraction for chromium speciation in human blood samples based on isopropyl 2-[(isopropoxycarbothiolyl) disulfanyl] ethane thioate, Anal. Chem. Res., 10 (2016) 18-27. https://doi.org/10.1016/j.ancr.2016.10.002
D. Zou, Y. Qing, Y. Li, M. Liu, Y. Yang, Determination of manganese (VII), chromium (VI) and nickel (II) in medicinal herb samples by cloud point extraction and high-performance liquid chromatography, J. Iran. Chem. Soc., 11 (2014) 415-422. https://doi.org/10.1007/s13738-013-0313-6
K. M. Diniz, C. R. T. Tarley, Speciation analysis of chromium in water samples through sequential combination of dispersive magnetic solid-phase extraction using mesoporous amino-functionalized Fe3O4/SiO2 nanoparticles and cloud point extraction, Microchem. J ., 123 (2015) 185-195. https://doi.org/10.1016/j.microc.2015.06.011
M. Rezvani, AA. Asgharinezhad, H. Ebrahimzadeh, N. Shekari, A polyaniline-magnetite nanocomposite as an anion exchange sorbent for solid-phase extraction of chromium (VI) ions, Microchim. Acta, 181 (2014) 1887-1895. https://doi.org/10.1007/s00604-014-1262-1
M. K. Abbasabadi, A. Rashidi, S. Khodabakhshi, Benzenesulfonic acid-grafted graphene as a new and green nanoadsorbent in hydrogen sulfide removal, J. Nat. Gas Sci. Eng., 28 (2016) 87-94. https://doi.org/10.1016/j.jngse.2015.11.043
J. Hummers, S. William, R. E. Offeman, Preparation of graphitic oxide, J. Am. Chem. Soc., 80 (1958) 1339. https://doi.org/10.1021/ja01539a017
M. K. Abbasabadi, D. Azarifar, Magnetic Fe3O4-supported sulfonic acid-functionalized graphene oxide (Fe3O4@ GO-naphthalene-SO3 H): a novel and recyclable nanocatalyst for green one-pot synthesis of 5-oxo-dihydropyrano [3, 2-c] chromenes and 2-amino-3-cyano-1, 4, 5, 6-tetrahydropyrano [3, 2-c] quinolin-5-ones, Res. Chem. Intermed., 45 (2019) 2095-2118. https://doi.org/10.1007/s11164-018-03722-y
R. M. Cespón-Romero, M. C. Yebra-Biurrun, Determination of trace metals in urine with an on-line ultrasound-assisted digestion system combined with a flow-injection preconcentration manifold coupled to flame atomic absorption spectrometry, Anal. Chim. Acta, 609 (2008) 184-191. https://doi.org/10.1016/j.aca.2008.01.002
T. Komatsu, M. Maeki, A. Ishida, H. Tani, M. Tokeshi, Based device for the facile colorimetric determination of lithium ions in human whole blood, ACS Sens., 5 (2020) 1287-1294. https://doi.org/10.1021/acssensors.9b02218
A. L. Suherman, B. Rasche, B. Godlewska, P. Nicholas, S. Herlihy, N. Caiger, P. J. Cowen, R. G. Compton, Electrochemical detection and quantification of lithium ions in authentic human saliva using LiMn2O4-modified electrodes, ACS sens., 4 (2019) 2497-2506. https://doi.org/10.1021/acssensors.9b01176
T. Filippini, B. Michalke, P. Grill, C. Malagoli, M. Malavolti, L. Vescovi, S. Sieri, V. Krogh, A. Cherubini, G. Maffeis, R. Lucchini, Determinants of serum manganese levels in an Italian population, Mol. Med. Rep., 15 (2017) 3340-3349. https://doi.org/10.3892/mmr.2017.6379
K. Zabłocka-Słowińska, S. Płaczkowska, A. Prescha, K. Pawełczyk, I. Porębska, M. Kosacka, L. Pawlik-Sobecka, H. Grajeta, Serum and whole blood Zn, Cu and Mn profiles and their relation to redox status in lung cancer patients, J. Trace Elem. Med. Biol., 45 (2018) 78-84. https://doi.org/10.1016/j.jtemb.2017.09.024
Copyright (c) 2021 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________