Determination and analysis of pesticide residues in fieldgrown and greenhouse-grown tomatoes using liquid chromatography-mass spectrometry
Volume 6, Issue 01, Pages 100-114, March 2023 *** Field: Analytical Method in Environmental Chemistry
Abstract
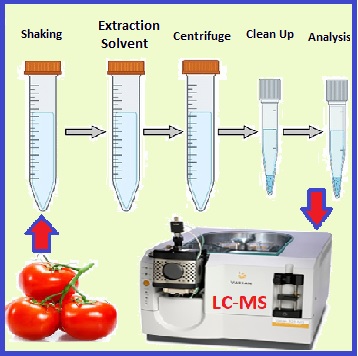
The present study aimed to extract pesticide residues in the field and greenhouse-grown tomatoes and homemade paste based on the (QuEChERS) method before being determined by liquid chromatography-mass spectrometry (LC-MS). The mean difference in percentage reduction of deltamethrin (DLM) and acetamiprid (ACT) in raw tomatoes of greenhouse-grown was obtained at 91.42 and 90.00%, respectively, which was insignificantly more than filed condition (84.91% and 86.34%). Maximum reduction percentages of the DLM in paste under greenhouse and field tomato conditions were achieved by more than 95.86% and 93.11%, respectively. The residual concentration of both DLM (91.42%) and ACT (90.00%) in the greenhouse decreased more than the field (84.91% and 86.34%), respectively. Abamectin(ABA) reached below the MRL in a shorter time after spraying (2 days). Considering the pre-harvest interval (PHI) period of deltamethrin and abamectin can reach their residual concentration to the MRL in both conditions, which were determined by LC-MS. According to the results of the current study, 7 and 5 days can be suggested as the PHI period of the acetamiprid for field and greenhouse-grown tomatoes, respectively. Therefore, using pesticides in the proper dosage, considering appropriate PHI, and harvesting can reduce their residues in agricultural products.
References
J. Mahugija, F. Ngabala, F. Ngassapa, Effectiveness of common household washing of tomatoes on the removal of pesticide residues, Tanzan. J. Sci, 47 (2021) 390-404. https://dx.doi.org/10.4314/tjs.v47i1.33.
K. Waheed, Medicinal Plants of South Asia, Elsevier, 2020. https://www.elsevier.com/books/medicinal-plants-of-south-asia/hanif/978-0-08-102659-5
A. Fghihi-Zarandi, F. Dabaghzadeh, A. Vaziri, S. Karami-Mohajeri, B. Ghorbaninejad, A. Zamani, K. Rahimi-Sadegh, Occupational risk assessment of organophosphates with an emphasis on psychological and oxidative stress factors, Toxicol. Ind. Health, 38 (2022) D13-064. https://doi.org/10.1177/07482337221096315.
L. Carrasco Cabrera, P. Medina Pastor, The 2020 European Union report on pesticide residues infood, Eu. Food Safety Authority J., 20 (2022) 7215. https://doi.org/10.2903/j.efsa.2022.7215
I. Ferrer, Multi-residue pesticide analysis in fruits and vegetables by liquid chromatography–time-of-flight mass spectrometry, J. Chromatogr. A, 1082 (2005) 81-90. https://doi.org/10.1016/j.chroma.2005.03.040.
H.N.R. Ratnamma, Determination and dissipation of acetamiprid using LC-MS/MS in okra, J. Entomol. Zool. Stud., 9 (2021) 110-116. https://www.entomoljournal.com/archives/2021/vol9issue1/PartB/8-6-198-106.pdf
A.Yazdanpak, Effects of washing, peeling and storage on residue contents of four pesticides in cucumbers grown in greenhouses (Cucumis sativus var:vista), J. anim. Environ., 12 (2020) 427-434. https://doi.org/10.22034/AEJ.2020.105708
M.E. Badawy, A.M. Ismail, A.I. Ibrahim, Quantitative analysis of acetamiprid and imidacloprid residues in tomato fruits under greenhouse conditions, J. Environ. Sci. Health B, 54 (2019) 898-905. https://doi.org/10.1080/03601234.2019.1641389.
A. Shekhi Gorjanet, Toxicity of some new generation insecticides against tomato leafminer moth, Tuta absoluta (Meyrick) under laboratory and greenhouse conditions, J. Appl. Res. Plant Prot., 7 (2018) 99-108. https://arpp.tabrizu.ac.ir/article_7498_en.html
J. Wang, H. Hirai, H. Kawagishi, Biotransformation of acetamiprid by the white-rot fungus Phanerochaete sordida YK-624, Appl. Microbiol. Biotechnol., 93 (2012) 831-835. https://doi. 10.1007/s00253-011-3435-8.
N.A. Ghalwa, M. Nasser, N. Farhat, Removal of abamectin pesticide by electrocoagulation process using stainless steel and iron electrodes, J. Environ. Anal. Chem., 2 (2015) 134. https://doi.org/10.4172/2380-2391.1000134
R.A. Nugroho, C. van Gestel, The acute single and mixture toxicity of paraquat dichloride and deltamethrin to Guppy (Poecillia reticulata), j. Biosci., 2 (2021) 47-53. https://doi.org/10.4308/hjb.29.1.47-53
A. Yazdanpak, H. Ostovan, Assessment of four pesticide residues (diazinon, imidacloprid, primicarb and acetamiprid) in cucumber under greenhouse condition of Iran (Fars province), J. Entomol. Res., 10 (2018) 139-148. https://jer.arak.iau.ir/article_665015_en.html
A.S. El Din, Persistence of acetamiprid and dinotefuran in cucumber and tomato fruits, J. Toxicol. Sci., 4 (2012) 103-107. https://doi. 10.5829/idosi.aejts.2012.4.2.1101.
S.K. Sahoo, Analysis of fluopicolide and propamocarb residues on tomato and soil using QuEChERS sample preparation method in combination with GLC and GCMS, Food Anal. Methods, 7 (2014) 1032-1042. https://doi. 10.1007/s12161-013-9709-2.
P. Komitet Normalizacyjny, Foods of plant origin Determination of pesticide residues using GC-MS and/or LC-MS/MS following acetonitrile extraction/partitioning and cleanup by dispersive SPE- QuEChERS-method, EN 15662, British Standard, 2008. http://www.chromnet.net/Taiwan/QuEChERS_Dispersive_SPE/QuEChERS_%E6%AD%90%E7%9B%9F%E6%96%B9%E6%B3%95_EN156622008_E.pdf
M.R. Rezaei Kahkha, A. Zarandi, N. Shafighi, S. Kosari, B. Rezaei Kahkha, Magnetic bentonite nanocomposite for removal of amoxicillin from wastewater samples using response surface methodology before determination by high performance liquid chromatography, Anal. Methods Environ. Chem. J., 3 (2020) 25-31. https://doi.org/10.24200/amecj.v3.i03.108.
A.A. Elbashir, A. Albadri, H.E. Ahmed, Effect of post-harvest and washing treatments on pesticide residues of fenpropathrin, λ-cyhalothrin, and deltamethrin applied on tomatoes grown in an open field in Sudan, Food Sci. Technol. Res., 2 (2013) 103-109. https://www.karger.com/Journal/Home/227093
R. Salghi, G. Luis, C. Rubio, A. Hormatallah, L. Bazzi, A. J. Gutiérrez, A. Hardisson, Pesticide residues in tomatoes from greenhouses in Souss Massa Valley, Morocco, Bull. Environ. Contam. Toxicol., 88 (2012) 358-361. https://doi.org/10.1007/s00128-011-0503-9.
B. Rafiei, S. Imani S. Bastan, Determination of residue of deltamethrin on greenhouse cucumber, J. Entomol. Res., 7 (2016) 307-316. https://jer.arak.iau.ir/article_522525.html?lang=en
R.M. Abdelfatahet, Dissipation of some pesticide residues in tomato (Lucopersicon esculentum L.) fruits using QuECHERS methodology under the Egyptian field conditions, J. Plant Prot. Pathol., 11 (2020) 327-332. https://doi.org/10.21608/jppp.2020.108835.
M.Fujita, Comparison of pesticide residue levels in headed lettuce growing in open fields and greenhouses, J. Pestic. Sci., 39 (2014) 13-064. https://doi.org/10.1584/jpestics.D13-064.
X. Chen, W. Wang , F. Liu, Y. Bian, Improved analysis of propamocarb and cymoxanil for the investigation of residue behavior in two vegetables with different cultivation conditions, J. Sci. Food Agric., 100 (2020) 3157-3163. https://doi.org/10.1002/%28ISSN%291097-0010.
N. Yigit, Y.S. Velioglu, Effects of processing and storage on pesticide residues in foods, Crit. Rev. Food Sci. Nutr., 60 (2020) 3622-3641. https://doi.org/10.1080/10408398.2019.1702501.
M.B. Medina, M.S. Munitz, S.L. Resnik, Effect of household rice cooking on pesticide residues, Food Chem., 342 (2021) 128311. https://doi.org/10.1016/j.foodchem.2020.128311.
A.A. Romehsup, M.Y. Hendawisup, Effect of processing on acetamiprid residues in eggplant fruits, Solanum melongena L, Afr. J. Agric. Res., 8 (2013) 2033-2037. https://doi.org/ 10.5897/AJAR2013.7240.
A. Hanafi, H.E. Elsheshetawy, S.F. Faied, Reduction of pesticides residues on okra fruits by different processing treatments, J. Verbrauch. Lebensm., 11 (2016) 337-343. https://doi.org/ 10.1007/s00003-016-1054-0
T.T. Nguyen, Fate of residual pesticides in fruit and vegetable waste (FVW) processing, Foods, 9 (2020) 1468. https://doi.org/10.3390/foods9101468.
M. Amirahmadi, Effect of Iranian traditional cooking on fate of pesticides in white rice, Toxin. Rev., 36 (2017) 177-186. https://doi.org/10.1080/15569543.2017.1301956.
L.Ajeep, Z. Alnaser, M.K. Tahla, Effect of household processing on removal of multi-classes of pesticides from tomatoes, J. Microbiol. Biotechnol. Food Sci., 10 (2021) 2015-2015. https://doi.org/10.15414/jmbfs.2015.
A. Shalaby, Health risk assessment of abamectin and buprofezin residues in eggplant and pepper plants, J. Plant Prot. Pathol., 11 (2020) 693-699. https://doi.org/10.21608/jppp.2020.166218.
A. Yazdanpak, Determination of residue levels of pesticides (acetamipride, diazinon, imidacloprid, primicarb) in greenhouse tomato (Solanum lycopersicum) var. Izmir in Fars, J. Anim. Environ, 11 (2019) 289-296. http://www.aejournal.ir/article_103902.html?lang=en
M. Osanloo, Validation of a new and cost-effective method for mercury vapor removal based on silver nanoparticles coating on micro glassy balls, Atm. Pollut. Res., 8 (2017) 359-365. https://doi.org/10.1016/j.apr.2016.10.004
M. Ghazaghi, H.Z. Mousavi, A. Rashidi, Ultrasound assisted dispersive micro solid-phase extraction of four tyrosine kinase inhibitors from serum and cerebrospinal fluid by using magnetic nanoparticles coated with nickel-doped silica as an adsorbent, Microchim. Acta, 183 (2016) 2779-2789. https://doi.org/10.1007/s00604-016-1927-z
AAM Beigi, MM Eskandari, B Kalantari, Dispersive liquid-liquid microextraction based on task-specific ionic liquids for determination and speciation of chromium in human blood, J. Anal. Chem., 70 (2015) 1448-1455. https://doi.org/10.1134/S1061934815120072
S. Golkhah, H. Zavvar Mousavi, Removal of Pb (II) and Cu (II) Ions from Aqueous Solutions by Cadmium Sulfide Nanoparticles, International Journal of Nanoscience and Nanotechnology 13 (2017) 105-117. http://www.ijnnonline.net/article_25609_8848b0eec7cbc60717bff650d460f600.pdf
H.Z. Mousavi, Chromium speciation in human blood samples based on acetyl cysteine by dispersive liquid–liquid biomicroextraction and in-vitro evaluation of acetyl cysteine/cysteine for decreasing of hexavalent chromium concentration, J. Pharm. Biomedical Analysis, 118 (2016) 1-8. https://doi.org/10.1016/j.jpba.2015.10.018
A. Khaligh, H.Z. Mousavi, A. Rashidi, Graphene oxide-packed micro-column solid-phase extraction combined with flame atomic absorption spectrometry for determination of lead (II) and nickel (II) in water samples, Int. J. Environ. Anal. Chem., 95 (2015) 16-32. https://doi.org/10.1080/03067319.2014.983437
Copyright (c) 2023 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________