A review: Total vaporization solid-phase microextraction procedure in different matrixes
Volume 5, Issue 03, Pages 80-102, Sep 2022 *** Field: Analytical Chemistry Review
Abstract
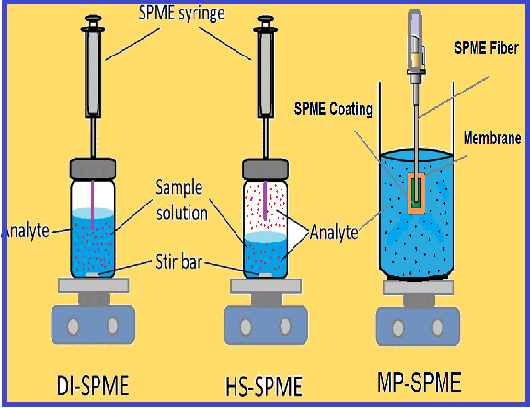
Total vaporization solid-phase microextraction (TV-SPME) is a type of extraction technique in which a specific solvent dissolves the analyte. Then a tiny amount of solvent is taken to the vial of SPME. Then, the solvent vaporizes in the SPME vial, and sampling is carried out on the headspace of the SPME fiber. As a result, the partitioning phase of the analyte between the headspace and liquid sample is omitted. The equilibrium phase remains the analyte partitioning between the headspace and SPME. TV-SPME was introduced in 2014 by Goodpaster to increase the recovery compared to the liquid injection method. This review discusses different aspects of TV-SPME, including its impact on sampling techniques, theoretical part, sampling procedure, and method optimization. Special attention was paid to its applications. A comprehensive literature study was conducted in the relevant databases to summarize the research work that has been done on this technique. In TV-SPME, the liquid samples completely vaporized and had a less matrix effect and better adsorption. This method needs no sample preparation, consumes less supply, and can be done automatically. Also, TV-SPME enables a cost-effective and efficient extraction for different matrixes. This review summarizes aspects related to TV-SPME.
References
C. L. Arthur, J. Pawliszyn, Solid phase microextraction with thermal desorption using fused silica optical fibers, Anal. Chem, 62 (1990) 2145-2148. https://doi.org/10.1021/ac00218a019.
M. Lashgari, V. Singh, J. Pawliszyn, A critical review on regulatory sample preparation methods: Validating solid-phase microextraction techniques, TrAC Trends Anal. Chem., 119 (2019). https://doi.org/10.1016/j.trac.2019.07.029.
E. Martendal, E. Carasek, A new approach based on a combination of direct and headspace cold-fiber solid-phase microextraction modes in the same procedure for the determination of polycyclic aromatic hydrocarbons and phthalate esters in soil samples, J. Chromatogr., A 1218 (2011) 1707-1714. https://doi.org/10.1016/j.chroma.2011.01.074.
E. Yiantzi, N. Kalogerakis, E. Psillakis, Vacuum-assisted headspace solid phase microextraction of polycyclic aromatic hydrocarbons in solid samples, Anal. Chim. Acta., 890 (2015) 108-116. https://doi.org/10.1016/j.aca.2015.05.047.
J. S. Elmore, M. A. Erbahadir, D. S. Mottram, Comparison of dynamic headspace concentration on Tenax with solid phase microextraction for the analysis of aroma volatiles, J. Agric. Food Chem. 45 (1997) 108-116. https://doi.org/10.1021/jf960835m.
S. A. Barshick, W. H. Griest, Trace analysis of explosives in seawater using solid-phase microextraction and gas chromatography/ion trap mass spectrometry, Anal. Chem., 70 (1998) 3015-3020. https://doi.org/10.1021/ac980060b.
S. A. Scheppers Wercinski, J. Pawliszyn, Solid Phase Microextraction Theory, Solid Phase Microextraction., Boca Raton, 257 pages, 1999. https://doi.org/10.1201/9780367803223
S. Huang, Monitoring of persistent organic pollutants in seawater of the Pearl River Estuary with rapid on-site active SPME sampling technique, Environ. Pollut, 200 (2015) 149-158. https://doi.org/10.1016/j.envpol.2015.02.016.
S. Risticevic., Protocol for solid-phase microextraction method development, Nat. Protoc., 5 (2010) 122-139. https://doi.org/10.1038/nprot.2009.179.
. É. A. Souza-Silva., A critical review of the state of the art of solid-phase microextraction of complex matrices III, Bioanalytical and clinical applications. TrAC Trends Anal. Chem., 71 (2015) 249-264. https://doi.org/10.1016/j.trac.2015.04.017.
S. Lv, The study of fingerprint characteristics of Dayi pu-erh tea using a fully automatic HS-SPME/GC-MS and combined chemometrics method, PLOS ONE, 9 (2014) e116428. https://doi.org/10.1371/journal.pone.0116428
C. L. Arthur, Automationandoptimizationofspme. Anal. Biochem., 64 (1992) 1960-1966. https://doi.org/10.1021/ac00041a034
J. Ai, Solid phase microextraction for quantitative analysis in nonequilibrium situations, Anal. Chem., 69 (1997) 1230-1236. https://doi.org/10.1021/ac9609541.
K. G. Furton, Application of solid-phase microextraction to the recovery of explosives and ignitable liquid residues from forensic specimens, J. Chromatogr., A 885 (2000) 419-432. https://doi.org/10.1016/s0021-9673(00)00368-x.
G. Liu, Headspace solid-phase microextraction of semi-volatile ultraviolet filters based on a superhydrophobic metal–organic framework stable in high-temperature steam, Talanta 219 (2020) 121175. https://doi.org/10.1016/j.talanta.2020.121175.
D. Bors, J. Goodpaster, Mapping explosive residues on galvanized pipe bomb fragments using total vaporization solid phase microextraction (TV-SPME), Anal. Methods, 7 (2015) 9756-9762. https://doi.org/10.1039/C5AY02358K.
R. Technologies, A review of: Static headspace – Gas chromatography theory and practice, J. Liq. Chromatogr. Relat. Technol., 21 (1998) 434-436, https://doi.org/10.1080/10826079808000503.
M. Tabibpour, Carbon fibers modified with polypyrrole for headspace solid phase microextraction of trace amounts of 2-pentyl furan from breath samples, J. Chromatogr. A, 1609(2020) 460497. https://doi.org/10.1016/j.chroma.2019.460497.
N. P. Brunton, D. A. Cronin, F. J. Monahan, The effects of temperature and pressure on the performance of Carboxen/PDMS fibres during solid phase microextraction (SPME) of headspace volatiles from cooked and raw turkey breast, Flavour Fragr. J. 16 (2001) 294-302. https://doi.org/10.1002/ffj.1000.
A. Mehdinia, M. O. Aziz-Zanjani, Advances for sensitive, rapid and selective extraction in different configurations of solid-phase microextraction, TrAC Trends Anal. Chem., 51 (2013) 13-22. https://doi.org/10.1016/j.trac.2013.05.013.
W. Q. Xie, Y. Gong, K. Yu, Enhancing the sensitivity of full evaporation technique using multiple headspace extraction analysis, Chromatographia, 80 (2017) 1263-1268. https://doi.org/10.1007/s10337-017-3343-x.
C. Wang, Oolong tea made from tea plants from different locations in Yunnan and Fujian, China showed similar aroma but different taste characteristics, Springerplus, 5 (2016) 576. https://doi.org/10.1186/s40064-016-2229-y.
W. Kranz, Optimization of total vaporization solid-phase microextraction (TV-SPME) for the determination of lipid profiles of Phormia regina, a forensically important blow fly species, Anal. Bioanal. Chem., 409 (2017) 6349-6357. https://doi.org/10.1007/s00216-017-0573-6.
J. L. Thomas, Application of a co-polymeric solid phase extraction cartridge to residues containing nitro-organic explosives, Forensic Chem., 11 (2018) 38-46. https://doi.org/10.1016/j.forc.2018.08.006.
S. Calderara, D. Gardebas, F. Martinez, Solid phase micro extraction coupled with on-column GC/ECD for the post-blast analysis of organic explosives, Forensic Sci. Int., 137 (2003) 6-12. https://doi.org/10.1016/s0379-0738(03)00256-1.
A. Jim, Application of solid-phase microextraction to Virgin olive oil quality control, J. Chromatogr A, 1121 (2006) 140-144. https://doi.org/10.1016/j.chroma.2006.05.005.
C. L. Rainey, D. E. Bors, J. V. Goodpaster, Design and optimization of a total vaporization technique coupled to solid-phase microextraction, Anal. Chem., 86 (2014) 11319-11325. https://doi.org/10.1021/ac5030528.
K. G. Furton, Application of solid-phase microextraction to the recovery of explosives and ignitable liquid residues from forensic specimens, J. Chromatogr. A, 885 (2000) 419-432. https://doi.org/10.1016/s0021-9673(00)00368-x.
M. A. LeBeau, M. A. Montgomery, M. L. Miller, S. G. Burmeister, Analysis of biofluids for gamma-hydroxybutyrate (GHB) and gamma-butyrolactone (GBL) by headspace GC-FID and GC-M, J. Anal. Toxicol., 24 (2000) 421-428. https://doi.org/10.1093/jat/24.6.421.
K. E. Davis, L. D. Hickey, J. V. Goodpaster, Detection of ɣ-hydroxybutyric acid (GHB) and ɣ-butyrolactone (GBL) in alcoholic beverages via total vaporization solid-phase microextraction (TV-SPME) and gas chromatography–mass spectrometry, J. Forensic Sci. 66 (2021) 846-853. https://doi.org/10.1111/1556-4029.14660.
D. N. Konanov, UniqPy: A tool for estimation of short-chain fatty acids composition by gas-chromatography/mass-spectrometry with headspace extraction, J. Pharm. Biomed. Anal., 212 (2022) 11468. https://doi.org/10.1016/j.jpba.2022.114681.
M. Beiranvand, Determination of BTEX compounds in contaminated water using the novel vacuum-assisted-total vaporization SPME method and GO-APTES fiber, J. Chromatogr. Sci., 60 (2022) 486-492. https://doi.org/10.1093/chromsci/bmab111.
K. E. Davis, J. V. Goodpaster, Gas chromatography-mass spectrometry paired with total vaporization solid-phase microextraction as a forensic tool, J. Vis. Exp., 171 (2021) 1-6. https://doi.org/10.3791/61880.
M. Llompart, M. Celeiro, C. García-Jares, T. Dagnac, Environmental applications of solid-phase microextraction, TrAC Trends Anal. Chem., 112 (2019) 1-12. https://doi.org/10.1016/j.trac.2018.12.020.
G. Sauzier, Optimisation of recovery protocols for double-base smokeless powder residues analysed by total vaporisation (TV) SPME/GC-MS, Talanta, 158 (2016) 368-374. https://doi.org/10.1016/j.talanta.2016.04.048.
L. Hickey, Automated derivatization and identification of controlled substances in solution via total-vaporization solid-phase microextraction (TV-SPME), SSRN Electron. J., (2021), https://doi.org/10.2139/ssrn.3888661.
O. M. Fayemiwo, M. O. Daramola, K. Moothi, Btex compounds in water – Future trends and directions for water treatment. Water South Africa, 43 (2017) 602-613. https://doi.org/10.4314/wsa.v43i4.08.
M. Rahmani, M. Kaykhaii, E. Ghasemi, M. Tahernejad, Application of in-syringe dispersive liquid–liquid microextraction and narrow-bore tube dispersive liquid–liquid microextraction for the determination of trace amounts of BTEX in water samples, J. Chromatogr. Sci., 53 (2015) 1210-1216, https://doi.org/10.1093/chromsci/bmu163.
M. Sajid, Porous membrane protected micro-solid-phase extraction: A review of features, advancements and applications, Anal. Chim. Acta., 965 (2017) 36-53. https://doi.org/10.1016/j.aca.2017.02.023.
M. Sajid, Development of natural sorbent based micro-solid-phase extraction for determination of phthalate esters in milk samples, Anal. Chim. Acta, 924 (2016) 35-44. https://doi.org/10.1016/j.aca.2016.04.016.
C. Basheer, H. K. Lee, Hollow fiber membrane-protected solid-phase microextraction of triazine herbicides in bovine milk and sewage sludge samples, J. Chromatogr., A 1047. (2004) 189-194. https://doi.org/10.1016/j.chroma.2004.06.130.
S. Merkle, K. Kleeberg, J. Fritsche, Recent developments and applications of solid phase microextraction (SPME) in food and environmental analysis—A review, Chromatogra. 2 (2015) 293-381. https://doi.org/10.3390/chromatography2030293
H. Piri-Moghadam, F. Ahmadi, J. Pawliszyn, A critical review of solid phase microextraction for analysis of water samples, TrAC Trends Anal. Chem., 85 (2016) 133-143. https://doi.org/10.1016/j.trac.2016.05.029.
X. Wang, Zinc(II)-based metal–organic nanotubes coating for high sensitive solid phase microextraction of nitro-polycyclic aromatic hydrocarbons, Talanta, 186 (2018) 561-567. https://doi.org/10.1016/j.talanta.2018.02.062.
M. Merdivan, V. Pino, J. L. Anderson, Determination of volatile polycyclic aromatic hydrocarbons in waters using headspace solid-phase microextraction with a benzyl-functionalized crosslinked polymeric ionic liquid coating, Environ. Technol., 38 (2017) 1897-1904. https://doi.org/10.1080/09593330.2016.1240242.
H. Mokbel, Simultaneous analysis of organochlorine pesticides and polychlorinated biphenyls in air samples by using accelerated solvent extraction (ASE) and solid-phase micro-extraction (SPME) coupled to gas chromatography dual electron capture detection, Environ. Sci. Pollut. Res. Int., 23 (2016) 8053-8063. https://doi.org/10.1007/s11356-016-6072-z.
M. Espina-Benitez, Development of a new microextraction fiber combined to on-line sample stacking capillary electrophoresis UV detection for acidic drugs determination in realwater samples, Int. J. Environ. Res. Public Health,, 14 (2017) 1-16. https://doi.org/10.3390/ijerph14070739.
R. López-Serna, Multiresidue analytical method for pharmaceuticals and personal care products in sewage and sewage sludge by online direct immersion SPME on-fiber derivatization – GCMS, Talanta, 186 (2018) 506-512. https://doi.org/10.1016/j.talanta.2018.04.099.
N. Ochiai, K. Sasamoto, A. Hoffmann, K. Okanoya, Full evaporation dynamic headspace and gas chromatography-mass spectrometry for uniform enrichment of odor compounds in aqueous samples, J. Chromatogr. A, 1240 (2012) 59-68. https://doi.org/10.1016/j.chroma.2012.03.097.
S. R. Kim, Method validation for measurement of hair nicotine level in nonsmokers, Biomed. Chromatogr., 23 (2009) 273-279. https://doi.org/10.1002/bmc.1110.
J. Kim, Association between secondhand smoke in hospitality venues and urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol concentrations in non-smoking staff, Int. J. Environ. Res. Public Health, 13 (2016) https://doi.org/10.3390/ijerph13111101.
C. L. Arthur, J. Pawliszyn, Solid phase microextraction with thermal desorption using fused silica optical fibers, Anal. Chem., 62 (1990) 2145-2148. https://doi.org/10.1021/ac00218a019,
J. You, BTEX degradation by a newly isolated bacterium: Performance, kinetics, and mechanism, Int. Biodeterior. Biodegrad., 129 (2018) 202-208. https://doi.org/10.1016/j.ibiod.2018.02.012.
N. De Giovanni, D. Marchetti, A systematic review of solid-phase microextraction applications in the forensic context, J. Anal. Toxicol., 44 (2020) 268-297. https://doi.org/10.1093/jat/bkz077.
D. M. Mana Kialengila, Full evaporation headspace gas chromatography for sensitive determination of high boiling point volatile organic compounds in low boiling matrices, J. Chromatogr. A, 1315 (2013) 167-175. https://doi.org/10.1016/j.chroma.2013.09.058.
N. Ochiai, K. Sasamoto, A. Hoffmann, K. Okanoya, Full evaporation dynamic headspace and gas chromatography-mass spectrometry for uniform enrichment of odor compounds in aqueous samples, J. Chromatogr. A, 1240 (2012) 59-68. https://doi.org/10.1016/j.chroma.2012.03.097.
E. Goudsmits, The analysis of organic and inorganic gunshot residue from a single sample, Forensic Sci. Int., 299 (2019) 168-173. https://doi.org/10.1016/j.forsciint.2019.03.049.
K. E. Davis, Detection of illicit drugs in various matrices via total vaporization solid-phase microextraction,AThesis,2019. https://scholarworks.iupui.edu/bitstream/handle/1805/19903/Purdue%20University%20Thesis%20Davis%202019%20FINAL.pdf?isAllowed=y&sequence=1
D. Bors, J. Goodpaster, Mapping smokeless powder residue on PVC pipe bomb fragments using total vaporization solid phase microextraction, Forensic Sci. Int., 276 (2017) 71-76. https://doi.org/10.1016/j.forsciint.2017.04.002.
Q. Guan, Rapid determination of total acid content of oils resulting from sub/supercritical water liquefaction of lignite by headspace gas chromatography, Energy Fuels, 27 (2013) 5135-5137. https://doi.org/10.1021/ef401020c.
M. Chan , H. Sy, Determination of ethanol content in kombucha using headspace gas chromatography with mass spectrometry detection: Single-laboratory validation, J. AOAC Int. 5 (2021) 122-128. https://doi.org/10.1093/jaoacint/qsaa094.
J. Schuberth, A. Full, A full Evaporation headspace technique with capillary GC and ITD: A means for quantitating volatile organic compounds in biological samples, J. Chromatogr. Sci., 34 (1996) 314-319. https://doi.org/10.1093/chromsci/34.7.314.
C. Pistos, J. T. Stewart, Direct injection HPLC method for the determination of selected benzodiazepines in plasma using a Hisep column, J. Pharm. Biomed. Anal., 33 (2003) 1135-1142. https://doi.org/10.1016/s0731-7085(03)00426-6.
J. Pawliszyn, S. Pedersen-Bjergaard, Analytical microextraction: Current status and future trends, J. Chromatogr. Sci., 44 (2006) 291-307. https://doi.org/10.1093/chromsci/44.6.291.
J. Pawliszyn, Theory of solid-phase microextraction, J. Chromatogr. Sci., 38 (2000) 270-278. https://doi.org/10.1093/chromsci/38.7.270
G. Vas, K. Vékey, Solid-phase microextraction: A powerful sample preparation tool prior to mass spectrometric analysi, J. Mass Spectrom., 39 (2004) 233-254. https://doi.org/10.1002/jms.606.
M. Beiranvand, A. Ghiasvand, Design and optimization of the VA-TV-SPME method for ultrasensitive determination of the PAHs in polluted water, Talanta, 212 (2020) 120809. https://doi.org/10.1016/j.talanta.2020.120809.
X. Sun, Detection of polycyclic aromatic hydrocarbons in water samples by annular platform-supported ionic liquid-based headspace liquid-phase microextraction, J. Anal. Methods Chem., 2018 (2018) 3765682. https://doi.org/10.1155/2018/3765682.
D. Bors, J. Goodpaster, Chemical analysis of racing fuels using total vaporization and gas chromatography mass spectrometry (GC/MS), Anal. Methods, 8 (2016) 3899-3902, https://doi.org/10.1039/C6AY00539J.
G. Sauzier, Optimisation of recovery protocols for double-base smokeless powder residues analysed by total vaporisation (TV) SPME/GC-MS, Talanta, 158 (2016) 368-374. https://doi.org/10.1016/j.talanta.2016.04.048.
C. Jurado et., Rapid analysis of amphetamine, methamphetamine, MDA, and MDMA in urine using solid-phase microextraction, direct on-fiber derivatization, and analysis by GC-MS, J. Anal. Toxicol., 24 (2000) 11-16. https://doi.org/10.1093/jat/24.1.11.
L. D. Hickey, Automated derivatization and identification of controlled substances via total vaporization solid phase microextraction (TV-SPME) and gas chromatography/mass spectrometry (GC/MS), Forensic & Investigative Science Program, thesis, 2019. http://dx.doi.org/10.7912/C2/2387
K. E. Davis, L. D. Hickey, J. V. Goodpaster, Detection of ɣ-hydroxybutyric acid (GHB) and ɣ-butyrolactone (GBL) in alcoholic beverages via total vaporization solid-phase microextraction (TV-SPME) and gas chromatography–mass spectrometry, J. Forensic Sci., 66 (2021) 846-853. https://doi.org/10.1111/1556-4029.14660.
A.V. de Bairros, Determination of low levels of benzodiazepines and their metabolites in urine by hollow-fiber liquid-phase microextraction (LPME) and gas chromatography–mass spectrometry (GC–MS), J. Chromatogra. B, 975 (2015) 24-33. https://doi.org/10.1016/j.jchromb.2014.10.040
G. S. Groenewold, J. R. Scott, C. Rae, Recovery of phosphonate surface contaminants from glass using a simple vacuum extractor with a solid-phase microextraction fiber, Anal. Chim., Acta, 697 (2011) 38-47. https://doi.org/10.1016/j.aca.2011.04.034.
E. Psillakis, Vacuum-assisted headspace solid-phase microextraction: A tutorial review, Anal. Chim., Acta, 986 (2017) 12-24, https://doi.org/10.1016/j.aca.2017.06.033.
N. Reyes-Garcés, Advances in solid phase microextraction and perspective on future directions, Anal. Chem., 90 (2018) 302-360. https://doi.org/10.1021/acs.analchem.7b04502.
M. Beiranvand, A. Ghiasvand, Simple, Low-cost and reliable device for vacuum-assisted headspace solid-phase microextraction of volatile and semivolatile compounds from complex solid samples, Chromatographia, 80 (2017) 1771-1780. https://doi.org/10.1007/s10337-017-3422-z.
A. Ghiasvand, F. Zarghami, M. Beiranvand, Ultrasensitive direct determination of BTEX in polluted soils using a simple and novel pressure-controlled solid-phase microextraction setup, J. Iran. Chem. Soc., 15 (2018) 1051-1059. https://doi.org/10.1007/s13738-018-1302-6.
R. Telles-Romero, Effect of temperature on pupa development and sexual maturity of laboratory Anastrepha obliqua adults, Bull. Entomol. Res., 101 (2011) 565-571. https://doi.org/10.1017/S0007485311000150.
B. Frere, GC-MS analysis of cuticular lipids in recent and older scavenger insect puparia. An approach to estimate the postmortem interval (PMI), Anal. Bioanal. Chem., 406 (2014) 1081-1088. https://doi.org/10.1007/s00216-013-7184-7.
J. E. Baker, D. R. Nelson, C. L. Fatland, Developmental changes in cuticular lipids of the black carpet beetle, Attagenus megatoma. Insect Biochem., 9 (1979) 335-339. https://doi.org/10.1016/0020-1790(79)90015-5.
M. Gołębiowski, Comparison of free fatty acids composition of cuticular lipids of Calliphora vicina larvae and pupae, Lipids, 47 (2012) 1001-1009. https://doi.org/10.1007/s11745-012-3702-1.
M. Gołębiowski, Cuticular and internal n-alkane composition of Lucilia sericata larvae, pupae, male and female imagines: Application of HPLC-LLSD and GC/MS-SIM, Bull. Entomol. Res., 102 (2012) 453-460. https://doi.org/10.1017/S0007485311000800.
M. Gołębiowski, The composition of the cuticular and internal free fatty acids and alcohols from Lucilia sericata males and females, Lipids, 47 (2012) 613-622. https://doi.org/10.1007/s11745-012-3662-5.
M. Gołębiowski, M. I. Boguś, M. Paszkiewicz, P. Stepnowski, Cuticular lipids of insects as potential biofungicides: Methods of lipid composition analysis, Anal. Bioanal. Chem., 399 (2011) 3177-3191. https://doi.org/10.1007/s00216-010-4439-4.
J. A. Yoder, G. J. Blomquist, D. L. Denlinger, Hydrocarbon profiles from puparia of diapausing and nondiapausing flesh flies (Sarcophaga crassipalpis) reflect quantitative rather than qualitative differences, Arch. Insect Biochem. Physiol., 28 (1995) 377-385. https://doi.org/10.1002/arch.940280407.
S. R. Kim, Method validation for measurement of hair nicotine level in nonsmokers, Biomed. Chromatogr., 23 (2009) 273-279. https://doi.org/10.1002/bmc.1110.
J. Kim, Association between secondhand smoke in hospitality venues and urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol concentrations in non-smoking staff, Int. J. Environ. Res. Public Health, 13 (2016)1101. https://doi.org/10.3390/ijerph13111101.
TWGFEX, Laboratory explosion group standards and protocols, Recommended guidelines for forensic identication of intact explosives, 2007. https://moodle.cranfield.ac.uk/pluginfile.php/408151/mod_resource/content/0/Guidelines_for_ident_of_post_blast_expl_residues-TWGFEX.pdf) (2007) 1-9.
M. A. Farajzadeh, N. Nouri, P. Khorram, Derivatization and microextraction methods for determination of organic compounds by gas chromatography, TrAC Trends Anal. Chem., 55 (2014) 14-23. https://doi.org/10.1016/j.trac.2013.11.006.
F. Pena-Pereira, I. Lavilla, C. Bendicho, Miniaturized preconcentration methods based on liquid–liquid extraction and their application in inorganic ultratrace analysis and speciation: A review, Spectrochim. Acta, B, 64(2009) 1-15. https://doi.org/10.1016/j.sab.2008.10.042.
R. M. Smith, Before the injection - Modern methods of sample preparation for separation techniques, J. Chromatogr. A, 1000 (2003) 3-27. https://doi.org/10.1016/s0021-9673(03)00511-9.
A. Balinova, R. Mladenova, D. Shtereva, Solid-phase extraction on sorbents of different retention mechanisms followed by determination by gas chromatography-mass spectrometric and gas chromatography-electron capture detection of pesticide residues in crops, J. Chromatogr. A, 1150 (2007) 136-144. https://doi.org/10.1016/j.chroma.2007.02.002.
J. M. DuBois, A mixed-method analysis of reports on 100 cases of improper prescribing of controlled substances, J. Drug Issues., 46 (2016) 457-472. https://doi.org/10.1177/0022042616661836.
M. Lashgari, Y. Yamini, An overview of the most common lab-made coating materials in solid phase microextraction, Talanta, 191 (2019) 283-306. https://doi.org/10.1016/j.talanta.2018.08.077.
T. O. Egbuchunam, G. Obi, F. E. Okieimen, F. Tihminlioglu, Removal of BTEX from aqueous solution using Organokaolinite, Int. J. Appl. Environ. Sci., 11 (2016) 505-513. http://www.ripublication.com
M. H. Dehghani, Source apportionment of BTEX compounds in Tehran, Iran using UNMIX receptor model, Air Qual. Atmos. Health, 10 (2017) 225-234, https://doi.org/10.1007/s11869-016-0425-0.
K. Khodaei, BTEX biodegradation in contaminated groundwater using a novel strain (Pseudomonas sp. BTEX-30), Int. Biodeterior. Biodegrad., 116 (2017) 234-242, https://doi.org/10.1016/j.ibiod.2016.11.001.
M. Hashemi, N. Jahanshahi, A. Habibi, Application of ultrasound-assisted emulsification microextraction for determination of benzene, toluene, ethylbenzene and o-xylene in water samples by gas chromatography, Desalination., 288 (2012) 93-97. https://doi.org/10.1016/j.desal.2011.12.017.
W. S. Khayoon, Micro-solid phase extraction with liquid chromatography-tandem mass spectrometry for the determination of aflatoxins in coffee and malt beverage, Food Chem., 147 (2014) 287-294, https://doi.org/10.1016/j.foodchem.2013.09.049.
F. Leusch, M. Bartkow, A short primer on benzene, toluene, ethylbenzene and xylenes (BTEX) in the environment and in hydraulic fracturing fluids, Smart Water Res. Cent., (2010) 1-8. https://www.ehp.qld.gov.au/management/coal-seam-gas/pdf/btex-report.pdf
Copyright (c) 2022 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________