An efficient cheap source of activated carbon as solid phases for extraction and removal of Congo Red from aqueous solutions
Volume 5, Issue 03, Pages 40-54, Sep 2022 *** Field: Analytical Chemistry in Environment
Abstract
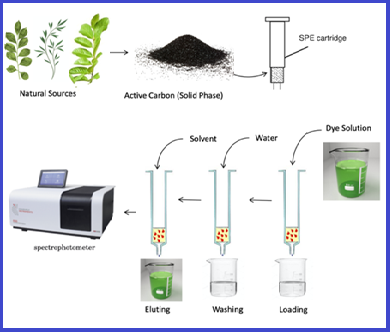
The present study reported the preparation of solid phases from various available and cheap natural sources represented by activated carbon to remove the polluting dye Congo Red (CR). Activated carbon derived from the leaves of the Consocarpus plant (C/AC) and Ziziphus Spina-Christi plant (Z/AC) and Myrtus plant (M/AC) by chemical activation. The solid phases were diagnosed and examined using FTIR, FESE, and XRD. The results of the study indicated that the best amount for the solid phase was 0.25 g for the three solid phases used against dye, the optimal concentration of the CR was 100 mg L-1, and the optimum acidity function was equal to 5 with a volume of 25 mL, as the optimization experiments indicated that the best flow rate of the eluting solution was equal to 0.5 ml min-1. The elution processes were carried out using several solvents different in polarity and it was found that 8 mL of DMSO achieved the best percentage of recovery (%R). Also, the adsorption capacity based on the optimal conditions that were obtained by applying Langmuir and Freundlich isotherm models, and qmax, according to the Langmuir model, was (21.74, 23.53, 22.17) mg g-1 for (Z/AC), (C/AC), and (M/AC) adsorbents, respectively.
References
X. Li, W. Bo, C. Yuhua, Z. Shuang, W. Hang, F. Xiao, Z. Junwen, M. Xiaojie, Water contaminant elimination based on metal–organic frameworks and perspective on their industrial applications, ACS Sustain. Chem. Eng., 7 (2019). 4548–4563. https://doi: 10.1021/acssuschemeng.8b05751.
N. Masoudian, M. Rajabi, M. Ghaedi, Titanium oxide nanoparticles loaded onto activated carbon prepared from bio-waste watermelon rind for the efficient ultrasonic-assisted adsorption of congo red and phenol red dyes from wastewaters, Polyhedron, 173 (2019) 114105. https://doi: https://doi.org/10.1016/j.poly.2019.114105.
K. Hossienzadeh, A. Maleki, H. Daraei, M. Safari, R. Pawar, S. M. Lee, Sonocatalytic and photocatalytic efficiency of transition metal-doped ZnO nanoparticles in the removal of organic dyes from aquatic environments, Korean J. Chem. Eng., 36 (2019) 1360–1370. https://doi: 10.1007/s11814-019-0299-6.
J. C. G. Sousa, A. R. Ribeiro, M. O. Barbosa, M. F. R. Pereira, A. M. T. Silva, A review on environmental monitoring of water organic pollutants identified by EU guidelines, J. Hazard. Mater., 344 (2018) 146–162. https://doi: 10.1016/j.jhazmat.2017.09.058.
M. A. Hassaan, A. El Nemr, Health and environmental impacts of dyes: Mini review, Am. J. Environ. Sci. Eng., 1 (2017) 64-67. https://doi: 10.11648/j.ajese.20170103.11.
K. Singh, S. Arora, Removal of synthetic textile dyes from wastewaters: A critical review on present treatment technologies, Crit. Rev. Environ. Sci. Technol., 41 (2011) 807–878. https://doi: 10.1080/10643380903218376.
E. Errais, Efficient anionic dye adsorption on natural untreated clay: Kinetic study and thermodynamic parameters, Desalination, 275 (2011) 74–81. https://doi.org/10.1016/j.desal.2011.02.031.
E. Zabłocka-Godlewska, W. Przystaś, E. Grabińska-Sota, Possibilities of obtaining from highly polluted environments: new bacterial strains with a significant decolorization potential of different synthetic dyes, Water Air Soil Pollut., 229 (2018)176. https://doi: 10.1007/s11270-018-3829-7.
M. S. El-Geundi, H. M. Ismail, K. M. E. Attyia, Activated clay as an adsorbent for cationic dyestuffs, Adsorpt. Sci. Technol., 12 (1995) 109–117. https://doi: 10.1177/026361749501200203.
S. A. Jadhav, Recent advancements in silica nanoparticles based technologies for removal of dyes from water, Colloid Interface Sci. Commun., 30 (2019) 100181. https://doi: https://doi.org/10.1016/j.colcom.2019.100181.
X. Meng, Synthesis, characterization, and utilization of a lignin-based adsorbent for effective removal of azo dye from aqueous solution, ACS omega, 5 (2020) 2865–2877. https://doi: 10.1021/acsomega.9b03717.
T. M. Budnyak, Methylene Blue dye sorption by hybrid materials from technical lignins, J. Environ. Chem. Eng., 6 (2018) 4997–5007. https://doi: 10.1016/j.jece.2018.07.041.
S. Banerjee, M. C. Chattopadhyaya, Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product, Arab. J. Chem., 10 (2017) S1629–S1638. https://doi: https://doi.org/10.1016/j.arabjc.2013.06.005.
M. B. Ahmed, J. L. Zhou, H. H. Ngo, W. Guo, N. S. Thomaidis, J. Xu, Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review, J. Hazard. Mater., 323 (2017) 274–298. https://doi.org/10.1016/j.jhazmat.2016.04.045.
Y. Zhou, J. Lu, Y. Zhou, Y. Liu, Recent advances for dyes removal using novel adsorbents: A review, Environ. Pollut., 252 (2019) 352–365. https://doi: 10.1016/j.envpol.2019.05.072.
J. Wang, Two new uncommon 3D cobalt-based metal organic frameworks: Temperature induced syntheses and enhanced photocatalytic properties against aromatic dyes, Dye. Pigment., 187 (2021), 109068. https://doi.org/10.1016/j.dyepig.2020.109068.
E. Dziurkowska, M. Wesolowski, Solid phase extraction purification of saliva samples for antipsychotic drug quantitation, Molecules, 23 (2018) 2946. https://doi: 10.3390/molecules23112946.
W. Jing, J. Wang, B. Kuipers, W. Bi, D. D. Y. Chen, Recent applications of graphene and graphene-based materials as sorbents in trace analysis, TrAC Trends Anal. Chem., 137 (2021) 116212. https://doi: https://doi.org/10.1016/j.trac.2021.116212.
M. He, L. Huang, B. Zhao, B. Chen, B. Hu, Advanced functional materials in solid phase extraction for ICP-MS determination of trace elements and their species - A review, Anal. Chim. Acta, 973 (2017) 1–24. https://doi: 10.1016/j.aca.2017.03.047.
M. H. Abdel-Aziz, DFT and experimental study on adsorption of dyes on activated carbon prepared from apple leaves, Carbon Lett., 31 (2021) 863–878. https://doi: 10.1007/s42823-020-00187-1.
H. Javadian, Using fuzzy inference system to predict Pb (II) removal from aqueous solutions by magnetic Fe3O4/H2SO4-activated Myrtus Communis leaves carbon nanocomposite, J. Taiwan Inst. Chem. Eng., 91 (2018) 186–199. https://doi: 10.1016/j.jtice.2018.06.021.
S. Farrokhzadeh, H. Razmi, B. Jannat, Application of marble powder as a potential green adsorbent for miniaturized solid phase extraction of polycyclic aromatic hydrocarbons from water samples, Sep. Sci. Technol., 55 (2020) 2737–2745. https://doi: 10.1080/01496395.2019.1655054.
J. Dai, Fabrication of novel ZIF-67 composite microspheres for effective adsorption and solid-phase extraction of dyes from water, Chem. Select, 3 (2018) 5833–5842. https://doi: https://doi.org/10.1002/slct.201800778.
H. S. Al-Niaeem, A. A. Abdulwahid, W. S. Hanoosh, Removal of carcinogenic dyes congo red (CR) and Bismarck brown Y (BBY) by adsorption onto reusable hydrogels derived from acrylamide, J. Phys. Conf. Ser., 2063 (2021) 012011. https://doi: 10.1088/1742-6596/2063/1/012011.
A. A. Mizhir, A. A. Abdulwahid, H. S. Al-Lami, Adsorption of carcinogenic dye congo red onto prepared graphene oxide-based composites, Desalin. Water Treat., 202 (2020) 381–395. https://doi: 10.5004/dwt.2020.26141.
A. A. Gouda, W. A. Zordok, Solid-phase extraction method for preconcentration of cadmium and lead in environmental samples using multiwalled carbon nanotubes, Turkish J. Chem., 42 (2018) 1018–1031. https://doi: 10.3906/kim-1711-90.
K. Hosseın, Mahdi and Dalalı, Nasser, Karımı, Ali and Dastanra, Solid phase extraction of copper, nickel, and cobalt in water samples after extraction using surfactant coated alumina modified with indane-1,2,3-trione 1,2-dioxime and determination by flame atomic absorption spectrometry, Turk. J. Chem., (2010), 805–814. https://doi.org/10.3906/sag-1203-108.
L. R. Snyder, Classification off the solvent properties of common liquids, J. Chromatogr. Sci., 16 (1978) 223–234. https://doi: 10.1093/chromsci/16.6.223.
Y. Li, W. Zhang, R.-G. Wang, P.-L. Wang, X.-O. Su, Development of a efficient and sensitive dispersive liquid–liquid microextraction technique for extraction and preconcentration of 10 β2-agonists in animal urine, PLOS One, 10 (2015) 1–16. https://doi: 10.1371/journal.pone.0137194.
R. Amer, H. Hadi, Application of CTAB-coated magnetic nanoparticles for solid-phase extraction of thiamine hydrochloride from pharmaceutical formulations and urine samples, Arab. J. Sci. Eng., 47 (2022) 429–440. https://doi: 10.1007/s13369-021-05671-y.
E. Yilmaz, G. Guzel Kaya, H. Deveci, Removal of methylene blue dye from aqueous solution by semi-interpenetrating polymer network hybrid hydrogel: Optimization through Taguchi method, J. Polym. Sci. Part A Polym. Chem., 57 (2019) 1070–1078. https://doi: https://doi.org/10.1002/pola.29361.
M. T. Nakhjiri, G. Bagheri Marandi, M. Kurdtabar, Adsorption of methylene blue, brilliant green and rhodamine B from aqueous solution using collagen-g-p(AA-co-NVP)/Fe3O4@SiO2 nanocomposite hydrogel, J. Polym. Environ., 27 (2019) 581–599. https://doi: DOI:101007/s10924-019-01372-8.
Y. Kuang, X. Zhang, S. Zhou, Adsorption of methylene blue in water onto activated carbon by surfactant modification, Water, 12 (2020) 587. https://doi: 10.3390/w12020587.
I. Langmuir, The constitution and fundamental properties of solids and liquids part 1 solids, J. Am. Chem. Soc., 38 (1916) 2221–2295. https://doi: 10.1021/ja02268a002.
A. A. Mizhir, A. A. Abdulwahid, H. S. Al-Lami, Chemical functionalization graphene oxide for the adsorption behavior of bismarck brown dye from aqueous solutions, Egypt. J. Chem., 63 (2020) 1679–1696. https://doi: 10.21608/ejchem.2020.21260.2271.
Wi. B. Meldrum, Experiments in physical chemistry, J. Chem. Educ., 28 (1951) 174. https://doi: 10.1021/ed028p174.3.
I. Crisan , R. Vidican, Phytoremediation Potential of Iris spp, Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Agric., 78 (2021) 1–10. https://doi: 10.15835/buasvmcn-agr:2020.0046.
P. Luo, B. Zhang, Y. Zhao, J. Wang, H. Zhang, J. Liu, Removal of methylene blue from aqueous solutions by adsorption onto chemically activated halloysite nanotubes, Korean J. Chem. Eng., 28 (2011) 800–807. https://doi: 10.1007/s11814-010-0426-x.
A. S. Muhammad, M. A. Abdurrahman, Adsorption of methylene blue onto modified agricultural waste, Moroccan J. Chem., 8 (2020) 412–427. https://doi.org/10.48317/IMIST.PRSM/morjchem-v8i2.16692.
Copyright (c) 2022 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________