Determine methylene blue based on carbon paste electrode modified with nanoparticles of nickel oxide-nitrogen carbon quantum dots and carbon structures by cyclic voltammetry
Volume 7, Issue 01, Pages 17-29, March 2024 *** Field: Analytical Chemistry
Abstract
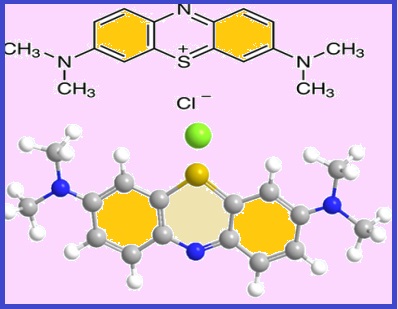
This paper deals with an electrochemical method for the determination of methylene blue (MB) by fabrication of an electrode based on a carbon paste modified with nano-nickel oxide and nitrogen carbon quantum dots (NiO-NCQD), graphene-carbon nitride (g-C3N4), reduced graphene oxide (rGO), and graphite powder and paraffin oil are as a plasticizer. This electrode is used as a working electrode. The analytical method used is cyclic voltammetry (CV), The oxidation-reduction curve of methylene blue was shown using this electrode. It is a quasi-reversible curve, and it works at (pH =1) and the best acid used is HCl a concentration of (0.1M). It was also found that the linear range is within the range of (7.99-31.98 mg L-1). The standard deviation (SD) and relative standard deviation (RSD%) were obtained at (0.361 mg L-1 and 0.294 mg L-1) and (4.52% and 3.68%) for both oxidation and reduction respectively. Retrospective, the limit of quantitative (LOQ) and limit of detection (LOD) were achieved at (99.65%; 99.70%), (0.24 mg L-1; 0.13 mg L-1), and (0.071 mg L-1; 0.039 mg L-1) for both oxidation and reduction respectively. Methylene blue was analyzed by UV-Vis spectrophotometry at (663 nm).
References
I. Khan, K. Saeed, I. Zekker, B. Zhang, A.H. Hendi, A. Ahmad, I. Khan, Review on methylene blue: its properties, uses, toxicity and photodegradation, Water, 14 (2022) 242. https://doi.org/10.3390/w14020242
H.N. Hamad, S. Idrus, Recent developments in the application of bio-waste-derived adsorbents for the removal of methylene blue from wastewater: A review, Polymers, 14 (2022) 783. https://doi.org/10.3390/polym14040783
T. Lupascu, M. Ciobanu, E. Culighin, Absorption of methylene blue from aqueous Solutions on activated coal CAN-9: kinetics and equilibrium studies, Roman. J. Ecol. Environ. Chem., 4 (2022) 22-28. https://doi.org/10.21698/rjeec.2022.102
A. Miclescu, L. Wiklund, L. Methylene blue, an old drug with new indications. J. Rom. Anest. Terap. Int., 17 (2010) 35-41. http://www.jurnalul-anestezie.ro/2010/h-Miclescu_Methylene%20blue.pdf
E.M. McDonagh, J.M. Bautista, I. Youngster, R.B. Altman, T.E. Klein, Pharm GKB summary: methylene blue pathway. Pharmacogenetics and genomics, 23 (2013) 498. https://doi.org/10.1097/FPC.0b013e32836498f4
C. Stack, S. Jainuddin, C. Elipenahli, M. Gerges, N. Starkova, A.A. Starkov, M. Dumont, Methylene blue upregulates Nrf2/ARE genes and prevents tau-related neurotoxicity, Hum. Mol. Genet., 23 (2014) 3716-3732. https://doi.org/10.1093/hmg/ddu080
M. Hassanpour, H. Safardoust-Hojaghan, M. Salavati-Niasari, Degradation of methylene blue and Rhodamine B as water pollutants via green synthesized Co3O4/ZnO nanocomposite, J. Mol. Liq., 229 (2017) 293-299. https://doi.org/10.1016/j.molliq.2016.12.090
V.K. Yadav, N. Choudhary, D. Ali, G. Kumar, G. Gnanamoorthy, A.U. Khan, B.Z. Tizazu, Determination of adsorption of methylene blue dye by incense stick ash waste and its toxicity on RTG-2 cells, Adsorp. Sci. Technol., 2022 (2022) 8565151. https://doi.org/10.1155/2022/8565151
Z. Reçber, Adsorption of methylene blue onto spent Alchemilla vulgaris leaves: Characterization, isotherms, kinetic and thermodynamic studies. Inter. J. Environ. Sci. Technol., 19 (2022) 4803–4814. https://doi.org/10.1007/s13762-022-04053-7
S.M. Merdas, W. Al-Graiti, A. Al-Ameer, (Using PVA@ WNS composite as adsorbent for methylene blue dye from aqueous solutions, J. Med. Chem. Sci., 5 (2022) 1289-1298. https://doi.org/10.26655/JMCHEMSCI.2022.7.18
A.E. Manlove, A.M. Linnebur, Primary bilateral cleft lip repair using the modified millard technique, Atlas Oral Maxillofac. Surg. Clin., 30 (2022) 19-25. https://doi.org/10.1016/j.cxom.2021.11.005
C. Luo, S. Wang, D. Wu, X., Cheng, H. Ren, UV/Nitrate photocatalysis for degradation of Methylene blue in wastewater: Kinetics, transformation products, and toxicity assessment, Environ. Technol. Innov., 25 (2022) 102198. https://doi.org/10.1016/j.eti.2021.102198
K. Duan, T. Que, S. Koppala, R. Balan, B. Lokesh, R. Pillai, S. Munusamy, A facile route to synthesize n-SnO 2/p-CuFe 2 O 4 to rapidly degrade toxic methylene blue dye under natural sunlight, RSC Adv., 12 (2022) 16544-16553. https://doi.org/10.1039/D2RA01690G
P.K. Gillman, CNS toxicity involving methylene blue: the exemplar for understanding and predicting drug interactions that precipitate serotonin toxicity, J. Psychopharm., 25 (2011) 429-436. https://doi.org/10.1177/0269881109359098
S.H. Mun, G.J. Park, J.H. Lee, Y.M. Kim, H.S. Chai, S.C. Kim, Two cases of fatal methemoglobinemia caused by self-poisoning with sodium nitrite: A case report, J. Med., 101 (2022) e28810. https://doi.org/10.1097/MD.0000000000028810
M. Oz, D.E. Lorke, G.A. Petroianu, Methylene blue and Alzheimer's disease, Biochem. Pharmacol., 78 (2009) 927-932. https://doi.org/10.1016/j.bcp.2009.04.034
A. Alemany, P. Millat-Martinez, M. Corbacho-Monné, P. Malchair, D. Ouchi, A. Ruiz-Comellas, E. Grau, High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial, Lancet Respir. Med., 10 (2022) 278-288. https://doi.org/10.1016/S2213-2600(21)00545-2
C. Jamshidzadeh, A new analytical method based on bismuth oxide-fullerene nanoparticles and photocatalytic oxidation technique for toluene removal from workplace air, Anal. Methods Environ. Chem. J. 2 (2019) 73-86. https://doi.org/10.24200/amecj.v2.i01.55
J.P. Tardivo, A. Del Giglio, C.S. De Oliveira, D.S. Gabrielli, H.C. Junqueira, D.B. Tada, M.S. Baptista, Methylene blue in photodynamic therapy: From basic mechanisms to clinical applications, Photodiagnosis Photodyn. Ther., 2 (2005) 175-191. https://doi.org/10.1016/S1572-1000(05)00097-9
M.T. Carneiro, A.Z. Barros, A.I. Morais, A.L. Carvalho Melo, R.D. Bezerra, J.A. Osajima, E.C. Silva-Filho, Application of water hyacinth biomass (Eichhornia crassipes) as an adsorbent for methylene blue dye from aqueous medium: Kinetic and isothermal study, Polymers, 14 (2022) 2732. https://doi.org/10.3390/polym14132732
M.M. Asl, N. Mansouri, Functionalized graphene oxide with bismuth and titanium oxide nanoparticles for efficiently removing formaldehyde from the air by photocatalytic degradation–adsorption process, J. Anal. Test., 7 (2023) 444-458. https://doi.org/10.1007/s41664-023-00272-0
A. El-Monaem, M. Eman, A.M. Omer, G.M. El-Subruiti, M.S. Mohy-Eldin, A.S. Eltaweil, Zero-valent iron supported-lemon derived biochar for ultra-fast adsorption of methylene blue, Biomass Convers. Bior., 14 (2024) 1697–1709. https://doi.org/10.1007/s13399-022-02362-y
V.Perumal, C. Inmozhi, R. Uthrakumar, R. Robert, M. Chandrasekar, S.B. Mohamed, K. Kaviyarasu, Enhancing the photocatalytic performance of surface-Treated SnO2 hierarchical nanorods against methylene blue dye under solar irradiation and biological degradation, Environ. Res., 209 (2022) 112821. https://doi.org/10.1016/j.envres.2022.112821
A. Nisar, M. Saeed, M. Muneer, M. Usman, I. Khan, Synthesis and characterization of ZnO decorated reduced graphene oxide (ZnO-rGO) and evaluation of its photocatalytic activity toward photodegradation of methylene blue, Environ. Sci. Pollut. Res., 29 (2022) 418-430. https://doi.org/10.1007/s11356-021-13520-6
S. Teimoori, New extraction of toluene from water samples based on nano-carbon structure before determination by gas chromatography, Int. J. Environ. Sci. Technol., 20 (2023) 6589-6608. https://doi.org/10.1007/s13762-023-04906-9
A.H. Hassani, M. Panahi, N. Mansouri, An immobilization of aminopropyl trimethoxysilane-phenanthrene carbaldehyde on graphene oxide for toluene extraction and separation in water samples, Chemosphere, 316 (2023)137800. https://doi.org/10.1016/j.chemosphere.2023.137800
O. Blank, E. Davioud-Charvet, M. Elhabiri, Interactions of the antimalarial drug methylene blue with methemoglobin and heme targets in Plasmodium falciparum: a physico-biochemical study, Antioxid. Redox Signal., 17 (2012) 544-554. https://doi.org/10.1089/ars.2011.4239
S. Roldán, M. Granda, R. Menéndez, R. Santamaría, C. Blanco, C. Supercapacitor modified with methylene blue as redox active electrolyte, Electrochim. Acta, 83 (2012) 241-246. https://doi.org/10.1016/j.electacta.2012.08.026
N. Martin, Y. Leprince-Wang, HPLC‐MS and UV–Visible coupled analysis of methylene blue photodegradation by hydrothermally grown ZnO nanowires, Phys. Status Solidi A, 218 (2021) 2100532. https://doi.org/10.1002/pssa.202100532
M.C. Henstridge, E. Laborda, E.J. Dickinson, R.G. Compton, Redox systems obeying Marcus–Hush–Chidsey electrode kinetics do not obey the Randles–Ševčík equation for linear sweep voltammetry, J. Electroanal. Chem., 664 (2012) 73-79. https://doi.org/10.1016/j.jelechem.2011.10.015
J.E.A. Randles, Cathode ray polarograph, Part II, the current-voltage curves, Trans. Faraday Soc., 44 (1948) 327-338. https://doi.org/10.1039/TF9484400327
A. García-Miranda Ferrari, C.W. Foster, P.J. Kelly, D.A. Brownson, C.E. Banks, Determination of the electrochemical area of screen-printed electrochemical sensing platforms, Biosensors, 8 (2018) 53. https://doi.org/10.3390/bios8020053
K.I. Alabid, H.N. Nasser, Study of the behavior and determination of phenol Based on modified carbon paste electrode with nickel oxide-nitrogen carbon quantum dots using cyclic voltammetry, Anal. Methods Environ. Chem. J., 6 (2023) 58-68. https://doi.org/10.24200/amecj.v6.i01.227
M.R. Jalali Sarvestani, R. Ahmadi, B. Farhang Rik, Procarbazine adsorption on the surface of single walled carbon nanotube: DFT studies, Chem. Rev. Lett., 3 (2020) 175-179. https://doi.org/10.22034/CRL.2020.110451
O.M.E.R. Rebaz, P. Koparir, L. Ahmad, M. Koparir, Computational determination the reactivity of salbutamol and propranolol drugs, Turk. Comput. Theor. Chem., 4 (2020) 67-75. https://doi.org/10.33435/tcandtc.768758
M.J. Sani, Spin-orbit coupling effect on the electrophilicity index, chemical potential, hardness and softness of neutral gold clusters: A relativistic ab-initio study, High Tech Innov. J., 2 (2021), 38-50. https://doi.org/10.28991/HIJ-2021-02-01-05
S. Erkan, Structural, spectroscopic and anti-cancer properties of hydroxy-and sulfonamide-azobenzene platinum (II) complexes: DFT and molecular docking studies, Cumhuriyet Sci. J., 39 (2018) 1036-1051. https://doi.org/10.17776/csj.421027
K.I. Alabid, H.N. Nasser, H.K. Maleh, Reduction of graphene oxide by new chemical and green methods, J. Ultrafine Grained Nanostruct. Mater., 55 (2022) 172-185. https://doi.org/10.22059/jufgnsm.2022.02.09
K.I. Alabid, H.N. Nasser, (2023). Synthesis and charcteriztion grapheme-carbon nitride nanostructure in one step, Ibn Al-haitham j. Pure Appl. Sci., 36 (2023) 260-272. https://doi.org/10.30526/36.3.3103.
K.I. Alabid, H.N. Nasser, Synthesis and characterization of Nickel Oxide with Nitrogen quantum Carbon dots as nanoadsorbent (NiO-NCQD) nanocomposite. Int. J. Nano Dimension, 14 (2023) 227-237. https://doi.org/10.22034/ijnd.2023.1984570.2217
Copyright (c) 2024 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________