Chromium desalinization using novel chitosan functionalized iron oxide- biochar composites: Analysis, synthesis, characterization and adsorption performance
Volume 7, Issue 01, Pages 30-48, March 2024 *** Field: Analytical Chemistry
Abstract
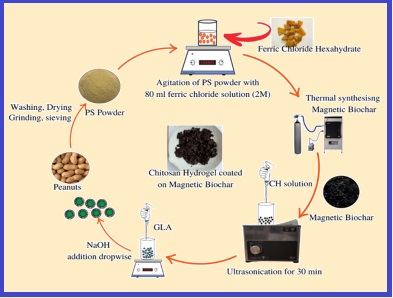
In the study, chitosan functionalized iron oxide incorporated with peanut shell biomass was prepared for potential adsorption of chromium (VI) from an aqueous media. The prepared material was characterized by modern spectroscopic methods for confirming the successful embedding. The adsorption experiments were conducted in batch systems. The experimental data showed robust removal of chromium supported by kinetic and equilibrium studies. The sorption data exhibited a strong agreement with the pseudo-second-order kinetics model, further confirming conformity with the Langmuir isotherm model. Adsorption studies were taken to find the effects of pH and time, reusability, ionic strength and presence of coexisting ions. The maximum sorption capacity was achieved as 14.28 mg g-1 at pH 4 and the optimum contact time was 40 minutes. The background electrolytes have much less effect on uptake efficiency and this green adsorbent can be utilized for up to four cycles. Additionally, a systematic approach was employed to ensure the precision and accuracy of the spectroscopic method. Calibration was linear in the range from 0.5 to 6.0 μg L-1 (R2 > 0.99). The limits of detection (LOD) and quantification (LOQ) were 0.65 μg L-1 and 2.16 μg L-1, respectively. The relative standard deviation (RSD) was 7.62 % (n=7).
References
L. Liu, W. Li, W. Song, M. Guo, Remediation techniques for heavy metal-contaminated soils: Principles and applicability, Sci. Total Environ., 633 (2018) 206-219. https://doi.org/10.1016/j.scitotenv.2018.03.161
X. Zhou, T. Korenaga, T. Takahashi, T. Moriwake, S. Shinoda, A Process monitoring controlling system for the treatment of wastewater containing chromium (VI), Water Res., 27 (1993) 1049-1054. https://doi.org/10.1016/0043-1354(93)90069-T
Y. Pang, Zeng, G.M. Zeng, L. Tang, Y. Zhang, Y. Y. Liu, X.X. Lei, M.S. Wu, Z. Li, C. Liu, Cr(VI) reduction by Pseudomonas aeruginosa immobilized in a polyvinyl alcohol/sodium alginate matrix containing multi-walled carbon nanotubes, Bioresour. Technol., 102 (2011) 10733-10736. https://doi.org/10.1016/j.biortech.2011.08.078
D. Mohan, C.U. Pittman, Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water, J. Hazard. Mater., 137 (2006) 762-811. https://doi.org/10.1016/j.jhazmat.2006.06.060
X. Guo, X. Lü, The need for biofuels in the context of energy consumption, Advances in 2nd generation of bioethanol production, Woodhead Publishing Series in Energy, CRC press, New York, pp 9-30, 2021. https://doi.org/10.1016/B978-0-12-818862-0.00004-2
W. Zheng, K. Phoungthong, F. Lü, L.M. Shao, P.J. He, Evaluation of a classification method for biodegradable solid wastes using anaerobic degradation parameters, Waste Manage., 33 (2013) 2632–2640. https://doi.org/10.1016/j.wasman.2013.08.015
I.G. Coronilla, L. M. Barrera, E. C. Urbina, Kinetic, isotherm and thermodynamic studies of amaranth dye biosorption from aqueous solution onto water hyacinth leaves, J. Environ. Manage., 152 (2015) 99-108. https://doi.org/10.1016/j.jenvman.2015.01.026
A. Zhang, X. Li, J. Xing, G. Xu, Adsorption of potentially toxic elements in water by modified biochar: A review, J. Environ. Chem. Eng., 8 (2020) 104196. https://doi.org/10.1016/j.jece.2020.104196
U. Upadhyay, I. Sreedhar, S.A. Singh, C.M. Patel, K. L. Anitha, Recent advances in heavy metal removal by chitosan based adsorbents, Carbohydr. Polym., 251(2021) 117000.
https://doi.org/10.1016/j.carbpol.2020.117000
E., Guibal, Interactions of metal ions with chitosan-based sorbents: A review, Sep. Purif. Technol., 38 (2004) 43-74. https://doi.org/10.1016/j.seppur.2003.10.004
W.C. Chong, J.F. Chin, Z.W. Heng, H.C. Teoh, Y.L. Pang, Recent development of magnetic biochar crosslinked chitosan on heavy metal removal from wastewater - Modification, application and mechanism, Chemosphere, 291 (2022)133035. https://doi.org/10.1016/j.chemosphere.2021.133035
J. Cheng, F. Xiao, W. Cao, C. Yang, J. Chen, Z. Luo, Removal of heavy metals from aqueous solution using chitosan-combined magnetic biochars, J. Colloid Interface Sci., 540 (2019) 579-584. https://doi.org/10.1016/j.jcis.2019.01.068
A. Choudhary, A. Kadawasara, S.S Poonia, P. kumar, V.K. Janu, Pyrolytic preparation of active carbons from peanut shell biomass for adsorptive elimination of fluoride from groundwater of Shekhawati region, Orient. J. Chem., 38 (2022) 1338-1350. http://dx.doi.org/10.13005/ojc/380602
APHA, Standard methods for the examination of water and wastewater. 21st edition, american public health association/American water works wssociation/water environment federation, Washington DC, 1288 pages, 2005. www.apha.org
M. Thompson, SLR. Ellison, R. Wood, Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC Technical Report). Pure Appl. Chem., 74 (2002) 835–855. https:// doi.org/10.1351/pac200274050835 35.
I. Langmuir, The constitution and fundamental properties of solids and liquids, J. Am. Chem. Soc., 183 (1917) 102–105. https://doi.org/10.1021/ja02268a002
H. Freundlich, Uber die adsorption in lösungen, Zeitschrift für Physikalische Chem., 57 A (1907) 385–470. https://doi.org/10.1515/zpch-1907-5723
S. Ahmed and S. Ikram, Chitosan and its derivatives: A Review in recent innovations, Int. J. Pharm. Res., 6 (2015) 14-30. http://dx.doi.org/10.13040/IJPSR.0975-8232.6(1).14-30
Y. S. Ho, G. McKay, The kinetics of sorption of divalent metal ions onto sphagnum moss peat, Water Res., 34 (2000) 735–742. https://doi.org/10.1016/S0043-1354(99)00232-8
S. Lagergren, On the theory of the so-called adsorption of dissolved substances, Solubility (Theoretical chemistry), Sven. Vetenskapsakad. Handingarl, 24 (1898) 1–39. https://cir.nii.ac.jp/crid/1570009750361875328
X. Duan, D. Wang, G. Qian, J.C. Walmsley, A. Holmen, D. Chen, X. Zhou, Fabrication of K-promoted iron/carbon nanotubes composite catalysts for the Fischer–Tropsch synthesis of lower olefins, J. Energy Chem., 22 (2016) 13-20. https://doi.org/10.1016/j.jechem.2016.01.003
J. Deng, Y. Liu, S. Liu, G. Zeng, X. Tan, B. Huang, X. Tang, S. Wang, Q. Hua, Z. Yan, Competitive adsorption of Pb(II), Cd(II) and Cu(II) onto chitosan-pyromellitic dianhydride modified biochar, J. Colloid. Interface. Sci., 506 (2017) 355-364. https://doi.org/10.1016/j.jcis.2017.07.069
R. Li, W. Liang, H. Huang, S. Jiang, D. Guo, M. Li, Z. Zhang, A. Ali, J.J. Wang, Removal of cadmium (II) cations from an aqueous solution with aminothiourea chitosan strengthened magnetic biochar, J. Appl. Polym. Sci., 135 (2018) 46239. https://doi.org/10.1002/app.46239
W. Zhang, S. Mao, H. Chen, L. Huang, R. Qiu, Pb(II) and Cr(VI) sorption by biochars pyrolyzed from the municipal wastewater sludge under different heating conditions, Bioresour. Technol., 147 (2013) 545–552. https://doi.org/10.1016/j.biortech.2013.08.082
F. Golbabaei, Z. Sadeghi, A. Vahid, A. Rashidi, On-line micro column preconcentration system based on amino bimodal mesoporous silica nanoparticles as a novel adsorbent for removal and speciation of chromium (III, VI), J. Environ. Health Sc. Eng., 13 (2015) 1-12. https://doi.org/10.1186/s40201-015-0205-z
M.M. Eskandari, B. Kalantari, Dispersive liquid-liquid microextraction based on task-specific ionic liquids for determination and speciation of chromium in human blood, J. Anal. Chem., 70 (2015) 1448-1455. https://doi.org/10.1134/S1061934815120072
H. Shirkhanloo, M. Ghazaghi, M.M. Eskandari, Cloud point assisted dispersive ionic liquid-liquid microextraction for chromium speciation in human blood samples based on isopropyl 2-[(isopropoxycarbothiolyl) disulfanyl, Anal. Chem. Res., 10 (2016)18-27. https://doi.org/10.1016/j.ancr.2016.10.002
H. Shirkhanlooa, M. Ghazaghi, H.Z. Mousavi , Chromium speciation in human blood samples based on acetyl cysteine by dispersive liquid–liquid biomicroextraction and in-vitro evaluation of acetyl cysteine/cysteine, J. Pharm. Biomed. Anal., 118 (2016) 1-8. http://dx.doi.org/10.1016/j.jpba.2015.10.018
S. A. H. Mirzahosseini, the evaluation and determination of heavy metals pollution in edible vegetables, water and soil in the south of Tehran province by GIS, Arch. Environ. Prot., 41 (2015) 63-72. https://doi.org/10.1515/aep-2015-0020
M Arjomandi, A review: analytical methods for heavy metals determination in environment and human samples, Anal. Methods Environ. Chem. J., 2 (2019) 97-126. https://doi.org/10.24200/amecj.v2.i03.73
V. Leitea, B. Jesisa, V. Duartea, V. Constantinob, C. Izumic, J. Trontoa, F. Pintoa, Determination of chromium (VI) by dispersive solid-phase extraction using dissolvable Zn-Al layered double hydroxide intercalated with L-Alanine as adsorbent, Microchem. J., 146 (2019) 650–657. https://doi.org/10.1016/j.microc.2019.01.063
A. Lace, D. Rayan, M. Bowkett, J. Cleary, Chromium Monitoring in water by colorimetry using optimised 1,5-diphenylcarbazide method, Int. J. Environ. Res. Public Health, 16 (2019) 1803; https://doi.org/10.3390/ijerph16101803
Copyright (c) 2024 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________