Solid phase-fabrication of multi-walled carbon nanotubes and their derivatives for efficient extraction and analysis of Bismarck Brown-Y Dye from aqueous solution
Volume 7, Issue 01, Pages 49-64, March 2024 *** Field: Analytical Chemistry
Abstract
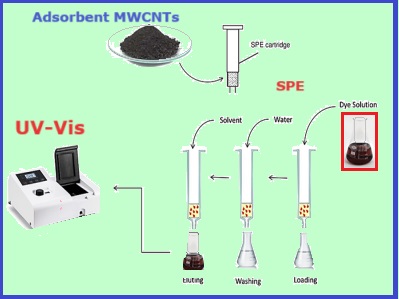
This investigation used efficientMWCNTs and their derivatives; MWCNT-Tris, MWCNT-H, MWCNT-Tetra and MWCNT-G, for extraction and removing the Bismarck Brown-Y (BB-Y) by solid phase extraction (SPE). The concentration of BB-Y was measured by UV-Vis spectrophotometer after the SPE technique. The solid phases were analyzed and characterized by utilizing several techniques, including Fourier Transform Infrared Spectroscopy (FTIR), FE-SEM, zeta potential measurement, and X-ray Diffraction (XRD). At optimization conditions, the optimum concentration of the BB-y was obtained at 200 mgL-1 and 300 mg L-1 for MWCNT and MWCNT-Tris, whereas 400 mg L-1 for MWCNT-H, MWCNT-Tetra, and MWCNT-G. Additionally, the optimal pH value was 6.0 for MWCNT-Tris, and it was 10 for MWCNT, MWCNT-H, MWCNT-Tetra, and MWCNT-G. However, the volume of samples was achieved at 25 mL. Furthermore, it was found that the most effective flow rate for the eluting solvent was 0.5 ml min-1. Besides the type and volume of eluents were examined and evaluated. Finally, the present work involved the determination of adsorption capacity using Langmuir and Freundlich isotherm models under ideal conditions. The Langmuir model revealed that the qmax for the MWCNT, MWCNT-tris, MWCNT-H, MWCNT-Tetra, and MWCNT-G was obtained 862.07, 1075.27, 1282.05, 1298.70, and 1333.33 mg g-1, respectively.
References
A. Balasubramanian, P. A. Balasubramanian, The world’s water geochemical modelling of groundwater for prevention of incrustation in the water supply systems of salem district, Tamil Nadu, Indian, View project educational video documentaries in earth, atmospheric and ocean sciences view project, 2017. https://www.researchgate.net/publication/315123891
P. Senthil Kumar, A critical review on recent developments in the low-cost adsorption of dyes from wastewater, Desalin. Water Treat., 172 (2019) 395–416. https://doi.org/ 10.5004/dwt.2019.24613
J. C. G. Sousa, A. R. Ribeiro, M. O. Barbosa, M. F. R. Pereira, A. M. T. Silva, A review on environmental monitoring of water organic pollutants identified by EU guidelines, J. Hazard. Mater., 344 (2018) 146–162. https://doi.org/10.1016/j.jhazmat.2017.09.058
F. F. A. Aziz, A. A. Jalil, S. Triwahyono, M. Mohamed, Controllable structure of fibrous SiO2 –ZSM5 support decorated with TiO2 catalysts for enhanced photodegradation of paracetamol, Appl. Surf. Sci., 455 (2018) 84–95. https://doi.org/10.1016/j.apsusc.2018.05.183
A. Kumar Samanta, P. Agarwal, Application of natural dyes on textiles, India. J. Fibre Texile Res., 34 (2009) 384-399. https://nopr.niscpr.res.in/bitstream/123456789/6886/1/IJFTR%2034(4)%20384-399.pdf
F. Mcyotto, Q. Wei, D. K. Macharia, M. Huang, C. Shen, C. W. K. Chow, Effect of dye structure on color removal efficiency by coagulation, Chem. Eng. J., 405 (2021) 126674. https://doi.org/10.1016/j.cej.2020.126674
Q. Wei, F. O. Mcyotto, C. W. K. Chow, Z. Nadeem, Z. Li, J. Liu, Eco-friendly decolorization of cationic dyes by coagulation using natural coagulant Bentonite and biodegradable flocculant sodium Alginate, SDRP J. Earth Sci. Environ. Stud., 5 (2020) 51–60. https://doi.org/ 10.25177/jeses.5.2.ra.10648
P. Arulmathi, C. Jeyaprabha, P. Sivasankar, V. Rajkumar, Treatment of textile wastewater by coagulation–flocculation process using gossypium herbaceum and polyaniline coagulants, Clean Soil, Air, Water, 47 (2019) 1800464. https://doi.org/10.1002/clen.201800464
N. Tara, S. I. Siddiqui, G. Rathi, S. A. Chaudhry, Inamuddin, A. M. Asiri, Nano-engineered adsorbent for the removal of dyes from water: A review, Curr. Anal. Chem., 16 (2019) 14–40. https://doi.org/10.2174/1573411015666190117124344
S. P. Keerthana, R. Yuvakkumar, P. S. Kumar, G. Ravi, D. V. N. Vo, D. Velauthapillai, Influence of tin (Sn) doping on Co3O4 for enhanced photocatalytic dye degradation, Chemosphere, 277 (2021) 130325. https://doi.org/10.1016/j.chemosphere.2021.130325
S. Varjani, P. Rakholiya, H. Y. Ng, S. You, J. A. Teixeira, Microbial degradation of dyes: An overview, Bioresour. Technol., 314 (2020) 123728. https://doi.org/10.1016/j.biortech.2020.123728
G. Crini, Non-conventional low-cost adsorbents for dye removal: A review, Bioresour. Technol., 97 (2006) 1061–1085. https://doi.org/10.1016/j.biortech.2005.05.001
V. K. Gupta, Suhas, Application of low-cost adsorbents for dye removal-A review, J. Environ. Manage., 90 (2009) 2313–2342 . https://doi.org/10.1016/j.jenvman.2008.11.017.
S. M. Ghoreishi, R. Haghighi, Chemical catalytic reaction and biological oxidation for treatment of non-biodegradable textile effluent, Chem. Eng. J., 95 (2003) 163–169. https://doi.org/10.1016/S1385-8947(03)00100-1
M. Rezaei, A. Habibi-Yangjeh, Simple and large scale refluxing method for preparation of Ce-doped ZnO nanostructures as highly efficient photocatalyst, Appl. Surf. Sci., 265 (2013) 591–596. https://doi.org/10.1016/j.apsusc.2012.11.053
B. Mu, A. Wang, Adsorption of dyes onto palygorskite and its composites: A review, J. Environ. Chem. Eng., 4 (2016) 1274–1294. https://doi.org/10.1016/j.jece.2016.01.036
X. Hou, S. Tang, J. Wang, J Wang, Recent advances and applications of graphene-based extraction, Trends Anal. Chem., 119 (2019) 115603. https://doi.org/ 10.1016/j.trac.2019.07.014
E. Dziurkowska, M. Wesolowski, Solid phase extraction purification of saliva samples for antipsychotic drug quantitation, Molecules., 23 (2018) 2946. https://doi.org/ 10.3390/molecules23112946
Y. Wen, L. Chen, J. Li, D. Liu, L. Chen, Recent advances in solid-phase sorbents for sample preparation prior to chromatographic analysis, TrAC Trends Anal. Chem., 59 (2014) 26–41. https://doi.org/10.1016/j.trac.2014.03.011
S. Di, Recent advances and applications of magnetic nanomaterials in environmental sample analysis, TrAC Trends Anal. Chem., 126 (2020) 115864. https://doi.org/10.1016/j.trac.2020.115864
C. Jamshidzadeh, A new analytical method based on bismuth oxide-fullerene nanoparticles and photocatalytic oxidation technique for toluene removal from workplace air, Anal. Methods Environ. Chem. J. 2 (2019) 73-86. https://doi.org/10.24200/amecj.v2.i01.55
M Arjomandi, A review: analytical methods for heavy metals determination in environment and human samples, Anal. Methods Environ. Chem. J., 2 (2019) 97-126. https://doi.org/10.24200/amecj.v2.i03.73
R. Ashouri, Dynamic and static removal of benzene from air based on task-specific ionic liquid coated on MWCNTs by sorbent tube-headspace solid-phase extraction procedure, Int. J. Environ. Sci. Technol., 18 (2021) 2377-2390. https://doi.org/10.1007/s13762-020-02995-4
J. Rakhtshah, A rapid extraction of toxic styrene from water and wastewater samples based on hydroxyethyl methylimidazolium tetrafluoroborate immobilized on MWCNTs by ultra-assisted dispersive cyclic conjugation-micro-solid phase extraction, Microchem. J., 170 (2021) 106759. https://doi.org/10.1016/j.microc.2021.106759
S. Teimoori, An immobilization of aminopropyl trimethoxysilane-phenanthrene carbaldehyde on graphene oxide for toluene extraction and separation in water samples, Chemosphere, 316 (2023) 137800. https://doi.org/10.1016/j.chemosphere.2023.137800
S. Teimoori, A.H. Hassani, M. Panahi, N. Mansouri, Rapid extraction of BTEX in water and milk samples based on functionalized multi-walled carbon nanotubes by dispersive homogenized-micro-solid phase extraction, Food Chem., 421 (2023) 136229. https://doi.org/10.1016/j.foodchem.2023.136229
S. Teimoori, A.H. Hassani, New extraction of toluene from water samples based on nano-carbon structure before determination by gas chromatography, Int. J. Environ. Sci. Technol., 20 (2023) 6589–6608. https://doi.org/10.1007/s13762-023-04906-9
B. Ali, Preparation of carbon nanotubes via chemical technique (modified staudenmaier method), Nanosci. Nanotechnol. Asia, 7 (2017) 113–122. https://doi.org/10.2174/2210681206666160711161421
Z. B. Ali Abdulnabi, H. T. Abdulsahib Faris, A J Al-doghachi, Synthesis and characterization of some selenazone complexes and nanoadsorbent surfaces from industrial waste for removing some carcinogenic dyes and heavy metals from water, proposal, 2021. https://faculty.uobasrah.edu.iq/uploads/publications/1643354925.pdf.
M. H. Abdel-Aziz, DFT and experimental study on adsorption of dyes on activated carbon prepared from apple leaves, Carbon Lett., 31 (2021) 863–878. https://doi.org/10.1007/s42823-020-00187-1
T. N. Majid, A. A. Abdulwahid, An efficient cheap source of activated carbon as solid phases for extraction and removal of Congo Red from aqueous solutions, Anal. Methods Environ. Chem. J., 5 (2022) 40–54. https://doi.org/10.24200/amecj.v5.i03.205
M. I. Mohammed, S. Baytak, Synthesis of bentonite–carbon nanotube nanocomposite and its adsorption of rhodamine dye From water, Arab. J. Sci. Eng., 41 (2016) 4775–4785. https://doi.org/ 10.1007/s13369-016-2190-7
G. Z. Kyzas, N. K. Lazaridis, Reactive and basic dyes removal by sorption onto chitosan derivatives, J. Colloid Interface Sci., 331 (2009) 32–39. https://doi.org/10.1016/j.jcis.2008.11.003
G. Z. Kyzas, D. N. Bikiaris, N. K. Lazaridis, Low-swelling chitosan derivatives as biosorbents for basic dyes, Langmuir., 24 (2008) 4791–4799. https://doi.org/10.1021/la7039064
A. A. Gouda, W. A. Zordok, Solid-phase extraction method for preconcentration of cadmium and lead in environmental samples using multiwalled carbon nanotubes, Turk. J. Chem., 42 (2018) 1018–1031. https://doi.org/10.3906/kim-1711-90
M. Hossein, N. Dalali, A. Karimi, K. Dastanra, Solid phase extraction of copper, nickel, and cobalt in water samples after extraction using surfactant coated alumina modified with indane-1, 2, 3-trione 1, 2-dioxime and determination by flame atomic absorption spectrometry, Turk. J. Chem., 34 (2010) 805–814. https://doi.org/10.3906/kim-1002-22
L. R. Snyder, Classification off the solvent properties of common liquids, J. Chromatogr. Sci., 16 (1978) 223–234. https://doi.org/10.1093/chromsci/16.6.223
Y. Li, W. Zhang, R. G. Wang, P. L. Wang, X. O. Su, Development of a efficient and sensitive dispersive liquid-liquid microextraction technique for extraction and preconcentration of 10 β2-Agonists in animal urine, PLOS One., 10 (2015) e0137194. https://doi.org/10.1371/journal.pone.0137194
G. Daneshvar Tarigh, F. Shemirani, Simultaneous in situ derivatization and ultrasound-assisted dispersive magnetic solid phase extraction for thiamine determination by spectrofluorimetry, Talanta., 123 (2014) 71–77. https://doi.org/10.1016/j.talanta.2014.01.045
E. Yilmaz, G. Guzel Kaya, H. Deveci, Removal of methylene blue dye from aqueous solution by semi-interpenetrating polymer network hybrid hydrogel: Optimization through Taguchi method, J. Polymer Sci., Part A: Polymer Chem.., 57 (2019) 1070–1078. https://doi.org/ 10.1002/pola.29361
Copyright (c) 2024 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________