Adsorption of methylene blue dye onto bentonite clay: Characterization, adsorption isotherms, and thermodynamics s tudy by using UV-Vis technique
Volume 6, Issue 03, Pages 5-18, Sep 2023 *** Field: Analytical Chemistry
Abstract
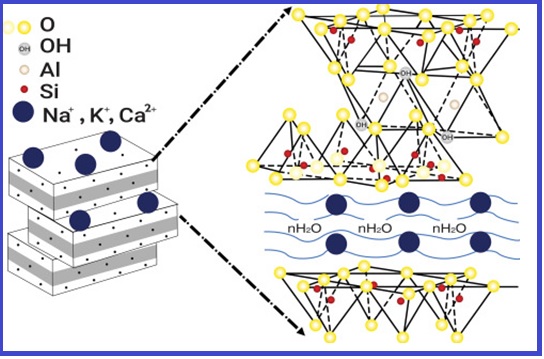
This study uses the UV-Vis technique to describe the elimination of methylene blue dye from an aqueous solution by adsorption on an Iraqi bentonite clay surface. The batch approach was used to conduct adsorption studies carried out to evaluate the influence of factors of experimental like contact time (0–90 min), clay dose (0.1–0.35 g), and initial dye concentration (10–125 mg L-1) at the range of temperatures (25-40oC). The Langmuir and the Freundlich isotherms were used to analyze the data; the Langmuir isotherm (R2 = 0.998) proved more appropriate for the equilibrium data. The thermodynamic properties of the adsorption process, including Gibbs free energy (ΔGO), entropy(ΔSO), and enthalpy (ΔHO), were also studied. Since the (ΔGO) and (ΔHO) values were negative, it was clear that the adsorption process constituted an exothermic, spontaneous reaction. This investigation revealed that Iraqi bentonite clay effectively removed the dye methylene blue because of its high surface area. Methylene blue may be removed with an adsorption efficiency of up to 99.39 % at 25oC. By employing bentonite clay as an adsorbent surface, this research offers practical adsorption technology that is affordable and effective for treating wastewater.
References
A.J. Ibrahim, ZnO nanostructure synthesis for the photocatalytic degradation of azo dye methyl orange from aqueous solutions utilizing activated carbon, Anal. Meth. Environ. Chem. J., 5 (2022) 5-19. https://doi.org/10.24200/amecj.v5.i04.200.
A. Debroy, M. Yadav, R. Dhawan, S. Dey, N. George, DNA dyes: toxicity, remediation strategies and alternatives, Folia Microbiol., 67 (2022) 555-571. https://doi.org/10.1007/s12223-022-00963-8.
F. Kooli, S. Rakass, Y. Liu, M. Abboudi, H.O. Hassani, S.M. Ibrahim, F. Wadaani, R. Al-Faze, Eosin removal by Cetyl trimethylammonium-Cloisites: influence of the surfactant solution type and regeneration properties, Mol., 24 (2019) 3015. https://doi.org/10.3390/molecules24163015.
G. A. Ismail, H. Sakai, Review on effect of different type of dyes on advanced oxidation processes (AOPs) for textile color removal, Chem., 291 (2022) 132906. https://doi.org/10.1016/j.chemosphere.2021.132906.
A. J. Ibrahim, Adsorption behavior of Crystal Violet dye in aqueous solution using Co+2 hectorite composite as adsorbent surface, Anal. Meth. Environ. Chem. J., 6 (2023) 5-16. https://doi.org/10.24200/amecj.v6.i01.219.
A.B. Fradj, A. Boubakri, A. Hafiane, S.B. Hamouda, Removal of azoic dyes from aqueous solutions by chitosan enhanced ultrafiltration, Res. Chem., 2 (2020) 100017. https://doi.org/10.1016/j.rechem.2019.100017.
M. E. Ali, M. E. Hoque, S. K. Safdar Hossain, M. C. Biswas, Nanoadsorbents for wastewater treatment: next generation biotechnological solution, Int. J. Environ. Sci. Technol., 17 (2020) 4095-4132. https://doi.org/10.1007/s13762-020-02755-4.
B. Bishayee, R. P. Chatterjee, B. Ruj, S. Chakrabortty, J. Nayak, Strategic management of nitrate pollution from contaminated water using viable adsorbents: An economic assessment-based review with possible policy suggestions, J. Environ. Manag., 303 (2022) 114081. https://doi.org/10.1016/j.jenvman.2021.114081.
E. A. Ashour, M. A. Tony, Eco-friendly removal of hexavalent chromium from aqueous solution using natural clay mineral: activation and modification effects, N. Appl. Sci., 2 (2020) 1-13. https://doi.org/10.1007/s42452-020-03873-x.
D. Dodoo, G. Appiah, G. Acquaah, T. D. Junior, Fixed-bed column study for the remediation of bauxite-liquid residue using acid-activated clays and natural clays, Heliyon, 9 (2023) e14310. https://www.cell.com/heliyon/pdf/S2405-8440(23)01517-7.pdf.
A. Awasthi, P. Jadhao, K. Kumari, Clay nano-adsorbent: structures, applications and mechanism for water treatment, SN. Appl. Sci. 1 (2019) 1076. https://doi.org/10.1007/s42452-019-0858-9.
W. Li, X. M. Liu, Experimental investigation of lithium isotope fractionation during kaolinite adsorption: Implications for chemical weathering, Geochim. Cosmochim. Acta, 284 (2020) 156-172. https://doi.org/10.1016/j.gca.2020.06.025.
I. M. Minisy, N. A. Salahuddin, M. M. Ayad, Adsorption of methylene blue onto chitosan–montmorillonite/polyaniline nanocomposite, App. Clay Sci., 203 (2021) 105993. https://doi.org/10.1016/j.clay.2021.105993.
Y.M. Vargas-Rodríguez, A. Obaya, J.E. García-Petronilo, G.I. Vargas-Rodríguez, A. Gómez-Cortés, G. Tavizón, J.A. Chávez-Carvayar, Adsorption studies of aqueous solutions of methyl green for halloysite nanotubes: Kinetics, isotherms, and thermodynamic parameters, Amer. J. Nanom., 9 (2021) 1-11. http://pubs.sciepub.com/ajn/9/1/1.
J. Matusik, A. Koteja-Kunecka, P. Maziarz, A. Kunecka, Styrene removal by surfactant-modified smectite group minerals: Efficiency and factors affecting adsorption/desorption, Chem. Eng. J., 428 (2022) 130848. https://doi.org/10.1016/j.cej.2021.130848.
U. Ortiz-Ramos, R. Leyva-Ramos, E. Mendoza-Mendoza, A. Aragón-Piña, Removal of tetracycline from aqueous solutions by adsorption on raw Ca-bentonite. Effect of operating conditions and adsorption mechanism, Chem. Eng. J., 432 (2022) 134428. https://doi.org/10.1016/j.cej.2021.134428.
S. Gao, D. Wang, Z. Huang, C. Su, M. Chen, X. Lin, Recyclable NiO/sepiolite as adsorbent to remove organic dye and its regeneration, Sci. Rep., 12 (2022) 2895. https://doi.org/10.1038/s41598-022-06849-6.
A.J. Ibrahim, Ultraviolet-activated sodium perborate process (UV/SPB) for removing humic acid from water, Anal. Meth. Environ. Chem. J., 5 (2022) 5-18. https://doi.org/10.24200/amecj.v5.i03.191.
J.A. Naser, T.A. Himdan, A.J. Ibraheim, Adsorption kinetic of malachite green dye from aqueous solutions by electrospun nanofiber Mat, Orient. J. Chem., 33 (2017) 3121-3129. http://dx.doi.org/10.13005/ojc/330654.
P. Pourhakkak, M. Taghizadeh, A. Taghizadeh, M. Ghaedi, Adsorbent, Inter. Sci. Tech., 33 (2021) 71-210. https://doi.org/10.1016/B978-0-12-818805-7.00009-6.
M. A. Rehman, T. Jafri, Stabilization of low plastic and high plastic clay using guar gum biopolymer, Int. J. Appl. Ind. Eng., 7 (2020) 329-343. http://dx.doi.org/10.22105/jarie.2020.247859.1195.
I. Chaari, E. Fakhfakh, M. Medhioub, F. Jamoussi, Comparative study on adsorption of cationic and anionic dyes by smectite rich natural clays, J. Mol. Stru., 1179 (2019) 672-677. https://doi.org/10.1016/j.molstruc.2018.11.039.
A. K. Dhar, H. A. Himu, M. Bhattacharjee, M. G. Mostufa, F. Parvin, Insights on applications of bentonite clays for the removal of dyes and heavy metals from wastewater: a review, Environ. Sci. Pollut. Res., 30 (2023) 5440-5474. https://doi.org/10.1007/s11356-022-24277-x
A.N.M.A. Haque, R. Remadevi, O.J. Rojas, X. Wang, M. Naebe, Kinetics and equilibrium adsorption of methylene blue onto cotton gin trash bioadsorbents, Cellulose, 27 (2020) 6485-6504. https://doi.org/10.1007/s10570-020-03238-y.
Y. Zhu, Y. Cui, Y. Peng, R. Dai, H. Chen, Y. Wang, Preparation of CTAB intercalated bentonite for ultrafast adsorption of anionic dyes and mechanism study, Colloids Surf. A., 658(2023) 130705. https://doi.org/10.1016/j.colsurfa.2022.130705.
H. Çiftçi, Removal of methylene blue from water by ultrasound-assisted adsorption using low-cost bentonites, Chem. Phys. Lett., 802 (2022) 139758. https://doi.org/10.1016/j.cplett.2022.139758.
A. Boukhemkhem, A. H. Pizarro, C. B. Molina, Enhancement of the adsorption properties of two natural bentonites by ion exchange: equilibrium, kinetics and thermodynamic study, Clay Mine., 55 (2020) 132-141. https://doi.org/10.1180/clm.2020.19.
J. Serafin, B. Dziejarski, Application of isotherms models and error functions in activated carbon CO2 sorption processes. Micro. Meso. Mat., 354 (2023) 112513. https://doi.org/10.1016/j.micromeso.2023.112513.
P. S. Kumar, G. J. Joshiba, C. C. Femina, P. Varshini, S. Priyadharshini, M. A. Karthick, R. Jothirani, A critical review on recent developments in the low-cost adsorption of dyes from wastewater, Desalin. Water Treat., 172 (2019) 395-416. https://doi.org/10.5004/dwt.2019.24613.
S. B. Denison, P. D. Da Silva, C. P. Koester, P. J. Alvarez, K. Zygourakis, Clays play a catalytic role in pyrolytic treatment of crude-oil contaminated soils that is enhanced by ion-exchanged transition metals, J. Hazard. Mater., 437 (2022) 129295. https://doi.org/10.1016/j.jhazmat.2022.129295.
Z. Tahr, A. Mohammed, J. A. Ali, Surrogate models to predict initial shear stress of clay bentonite drilling fluids incorporated with polymer under various temperature conditions, Arab J. Geosci., 15 (2022) 1449. https://doi.org/10.1007/s12517-022-10720-3.
X. Guo, J. Wang, Comparison of linearization methods for modeling the Langmuir adsorption isotherm, J. Mol. Liq., 296 (2019) 111850. https://doi.org/10.1016/j.molliq.2019.111850.
E. R. Orhue, A. Emomu, Freundlich, Langmuir and Temkin isotherm studies of silicon sorption on soils derived from three parent materials in Edo State, Agro-Sci., 21 (2022) 1-12. https://doi.org/10.4314/as.v21i3.1.
E. Y. Soh, S. S. Lim, K. W. Chew, X. W. Phuang, V. M. Ho, K. Y. Chu, R. R. Wong, L. Y. Lee, T. J. Tiong, Valorization of spent brewery yeast biosorbent with sonication-assisted adsorption for dye removal in wastewater treatment, Environ. Res., 204 (2022) 112385. https://doi.org/10.1016/j.envres.2021.112385.
E. C. Lima, A. A. Gomes, H. N. Tran, Comparison of the nonlinear and linear forms of the van't Hoff equation for calculation of adsorption thermodynamic parameters (∆ S° and ∆ H°), J. Mol. Liq., 311 (2020) 113315. https://doi.org/10.1016/j.molliq.2020.113315.
L. Q. Chen, Chemical potential and Gibbs free energy, MRS. Bulletin., 44 (2019) 520-523. https://doi.org/10.1557/mrs.2019.162.
I. Tahira, Z. Aslam, A. Abbas, M. Monim-ul-Mehboob, S. Ali, A. Asghar, Adsorptive removal of acidic dye onto grafted chitosan: a plausible grafting and adsorption mechanism, Int. J. Biol. Macromol., 136 (2019) 1209-1218. https://doi.org/10.1016/j.ijbiomac.2019.06.173.
Copyright (c) 2023 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________